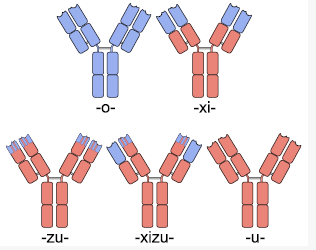
Globally, the monoclonal antibodies market has emerged as a major money spinner for most companies. Indian companies are also tapping this potential market but the common issue faced by them is related to investment and process complexity

Growth demand for mAbs
With dwindling R&D pipelines, patent expiration of blockbuster products, and apprehensions over subsequent dip in revenues till 2012, most pharmaceutical companies have chalked out a ‘mAbs strategy’. This was evident from a spate of licensing deals between pharma and biotech companies in the mAbs space and a spree of biotech firms being acquired by pure pharma companies in the recent past. In 2006, GSK etched a mark with the company licensing a drug for leukaemia from Genmab for Rs 9,341 crore ($2.1 bn), the largest ever deal in terms of value in the mAbs segment. The year 2009 saw some mega mergers with the Roche-Genentech deal gaining the center of attraction.
“MAbs offer an enormous amount of target specificity that reduces the nonspecific, untoward side effects commonly observed in small molecules. Many companies across the globe have mAbs products in advanced clinical trials stage,” says Dr Mody. Cancer and anti-infammatory segments contribute 51 and 30 percentages respectively, according to a report by Datamonitor and Frost & Sullivan.
Experts from the scientific community point out that human mAb has a higher rate of technical success and negligible levels of toxicity and lesser degrees of side effects. “The platform technology can easily be adapted for novel mAbs as the variability that can be introduced within the antigen-binding domains of mAbs increases their molecular diversity and extends the range of potential therapeutic applications,” says Dr Mody.
On the international front, numerous factors have motivated the companies to opt for mAbs. There has been an upsurge in demand for mAbs products because very few chemical-based products are available to provide effective cure for diseases such as cancer, asthma, anti-inflammatory, osteoporosis and opthalmology. “Chemical-based products have failed to provide remedy for oncology diseases. The technological competency of mAbs helps to get rid of these diseases,” says Sujay Shetty, associate director of pharmaceutical and lifesciences, PricewaterhouseCoopers India.
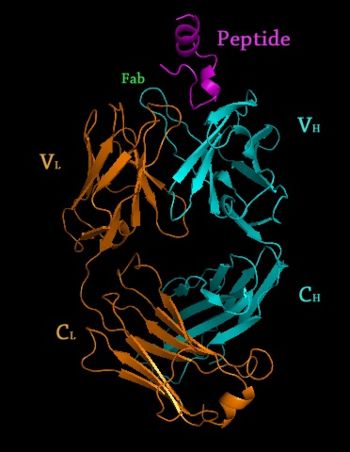
Crystal Structure of Rituximab Fab in complex with an epitope peptide 2osl
The commercial success of blockbuster products like Avastin and Herceptin for oncology; Humira and Remicade for AIID; and Rituxan for both oncology and AIID, has been a stimulus for the companies rolling out a large number of R&D projects in mAbs. Remicade has been the best selling antibody since 2004 and was the market leader with sales of over Rs 28,915 crore ($6.5 bn) in 2008, followed by Rituxan, Avastin, Herceptin and Humira. The first three products made it to the top ten and all five products made it to the top 20 global best selling human medicinal brands. Till 2008, there were over 200 antibodies out of 630 biologics in clinical trials testing for cancer, arthritis, infections, asthma, macular degeneration, osteoporosis, diabetes and other chronic diseases. Increased sales and profitability have attracted mainstream pharmaceutical companies towards mAbs. The pressure of pricing is much lower in this space.
In India, the demand for mAbs is increasing and there is an upsurge in the number of Indian companies venturing into this space. India has capitalized on its so-called ‘low cost destination’ advantage. “Huge investment is needed to establish large scale manufacturing facilities for mAbs. The fund needed for setting up large scale operations is still lower in India as compared to developed markets. By establishing their operations in India those developed markets can fulfill their growing demand for mAbs products. India offers the possibility of improving their profit margins,” says Dr Mody . Biocon and Dr Reddy’s Labs, two of India’s top life sciences companies, have already launched their products in the market successfully.
The latest debate among the biotech circles are the opportunities that biosimilar mAbs has to offer to companies. The global biosimilars market is primarily dominated by three main components – mAbs, therapeutic proteins and vaccines. In 2009, 29 mAbs were approved and marketed for therapeutic use. With the patent expiry of products like Herceptin, Humira and Rituxan by 2020, generic versions of these products are in the pipeline of many Indian players. Analysts predict that it will be a tussle for dollars for Indian players in the coming decade. Currently there are about 25 Indian companies operating in the space bringing out at least 40 products in the market and many of them are well positioned to compete in the global mAbs landscape. “This product class is gaining maturity and within five years, when the second wave of biologicals are going to be off-patent, many of which are blockbuster mAbs, India is likely to dominate biosimilar mAbs development and manufacturing,” adds Dr Mody.
In terms of funding, venture capital firms are optimistic about mAbs space. “Globally, venture capitalists are willing to invest in two areas – interferons and mAbs,” says Shetty The main reason for this is the patent expiry of mAbs worth Rs 47,154 crore ($10.6 bn) by 2018. MAbs is the focus area for many pharma and non-pharma companies and hence R&D, M&A and licensing deals are happening in billion dollars for this class of products. A venture capitalist from a leading firm in India told BioSpectrum (without mentioning any names) that he was aware of many reputed VC firms (which includes his firm) in India are looking at investing in mAbs space in the event of a rising number of Indian companies venturing into the segment.
mAbs in India
Biocon was the first Indian company to come up with its mAbs product, BIOMAb-EGFR, and the product was granted regulatory marketing and manufacturing approval in India in September 2006. The product is a therapeutic monoclonal antibody-based drug for treating solid tumors of epithelial origin, such as head and neck cancers. This novel drug is engineered to specifically target and block the epidermal growth factor receptor (EGFR) responsible for the proliferation of cancer cells. Dr Harish Iyer, R&D head, Biocon, “As far as Biocon’s portfolio is concerned, we have one product in the market from our stable.
BIOMAb EGFR (Nimotuzumab) is a monoclonal antibody that specifically binds to the extracellular domain of EGFR and prevents signal transduction. It is used in the treatment of advanced squamous cell carcinoma of the head and neck region with concurrent chemotherapy and/or radiotherapy.” It is also being globally studied in a range of solid tumor types, including colorectal cancer, lung cancer, glioma and pancreatic cancer. “Biocon has also partnered with Mylan for co-developing biosimilar mAbs. This is a co-development, cost sharing agreement for a bunch of molecules that are currently in development,” says Dr Iyer.

Apart from being a low cost manufacturing destination, Indian companies have the upper hand of offering mAbs products at comparatively lesser price margins. At the launch of BIOMAb -EGFR, Dr Kiran Mazumdar-Shaw, chairman and MD of Biocon, said, “BIOMAb -EGFR is competitively priced to make cancer treatment more affordable. “In 2007, Dr Reddy’s Labs came out with the novel concept of producing the biosimilar version of Rituximab, a version of Roche’s cancer therapy, which could allow a greater access to the drug at half the price of the original. With the more complex molecules like mAbs, Dr Reddy’s believes that the preferred strategy is to systematically develop the entire spectrum of development and manufacturing capabilities. The complexity of the molecules and the processes means a close integration of all the relevant skills within one organization with direct links between the manufacturing groups and the process, analytical, pre-clinical and clinical development groups. World-class facilities and laboratories of Dr Reddy’s, the scientific depth of its team, the robustness of the development strategy and the focus on quality issues were some of the key factors that contributed to the successful development of a complex molecule like Reditux.
Reditux is the second product from Dr Reddy’s Biologics Division, which is developing treatments for cancer and autoimmune diseases. The company has also launched the generic version of Amgen’s Neupogen, and named it Grafeel. The company has spent more than Rs 44.48 crore ($10 mn) for developing Reditux and within a year of its launch the products have successfully gained 30-35 percent share of the market. Dr Reddy’s Reditux is priced at Rs 39,996 for a vial and is almost half the price of Roche’s Mabthera. This product is now approved for marketing in India.
Pune-based Serum Institute has entered into another agreement with Akorn of the US in 2007 for definitive development and exclusive distribution rights for rabies mAb. As part of the agreement, Serum has agreed to appoint Akorn as the exclusive distributor for rabies mAb. In exchange for Akorn receiving the exclusive marketing and distribution rights of North, Central, and South America, Akorn has agreed to provide Serum funding for product development through milestone payments.
Intas Biopharmaceuticals has signed a Memorandum of Understanding (MoU) with Government of Gujarat in 2009 for setting up a separate manufacturing facility for MAbs, a recombinant mammalian platform product. The company will invest Rs 160 crore towards setting up a manufacturing facility at Sanand near Ahmedabad. The facility, fully-dedicated for mAbs, will undertake large-scale manufacturing of the recombinant product with a capacity of 5000L in phases. “As part of its Strategic Research Initiative (SRI), the company is focusing on cloning mAbs and developing proprietary and novel expression systems using different mammalian cell lines,” says Dr Mody.
These companies apart, Daftary group promoted Bharat Serums and Vaccines and Bangalore-based Avesthagen are some of the other notable companies that have chalked our serious plans for the space.
Challenges ahead
MAbs are complex protein molecules. In addition to the protein structure, often there is a carbohydrate moiety attached to the molecule. Characterization of such complex molecules is a challenge, this is why, very few companies in India are active in mAbs arena. “Not only do you need sophisticated equipment to analyze mAbs, you also need skilled manpower to do this. Since most of these are immune-modulatory in nature, they have complex reactions with the human body.
Therefore, we have to determine the biological activity of these molecules using specific cell lines. Performing such bioassays is also a challenging,” adds Dr Iyer.
Industry experts agree that the mAbs market in India is in its nascent stage. In India, only few players are active in mAbs space. There are many players who have aspirations to enter the space as the next generation of biotech products would be mAbs. However, there are only a handful of companies that have products in the preclinical stage.
The reasons are many. “It is difficult to copy human mAbs, which is complex and expensive process. It takes a long time to develop and it needs some vigorous ground work and intensive research that Indian companies are yet to gain mastery,” adds Shetty.
In addition to this, the Drug Controller General of India (DCGI) is yet to come out with a set of systematic guidelines dedicated especially for mAbs. Companies like Dr Reddy’s Labs and Biocon have been the only two companies successful in bringing in products while the remaining are still in the development stage.”Shetty says, “It will take some time for India to become a lucrative market for mAbs. In biogenerics, too, the market would take time to pick up. In addition to this, the high costs of investments involved can also be a barrier.
“MAbs business involves patents and for navigating through these patents requires exceptional skills,” adds Dr Mody.
Future prospects
By 2015 , due to the expected launch of many new therapies such as Denosumab and Teplizumab, the sales of mAbs is expected to reach Rs 3 lakh crore ($67.6 bn), with a CAGR of 13.8 percent between 2008 and 2015. The biotherapeutic market would be dominated with mAbs in next five years as most of the blockbuster molecules are going off-patented.
All the pharma majors have mAbs projects in their R&D portfolio. Introduction of newer monoclonal antibodies will greatly expand the market. Globally, the FDA had approved four new mAbs and EMA had approved seven new mAbs in 2009. There were at least six new mAbs under regulatory review, 26 mAbs (32 in 2008) are in phase III and over 100 are in phase II clinical trials. There are more than 150 mAbs molecule awaiting regulatory approval. In addition to this, several promising candidates from companies like Pfizer are in phase III clinical trials.
In the recent past, Sanofi-aventis Chris Viehbacher, CEO of Sanofi-aventis, had commented that they had missed the ‘boat to biologics’ and are in active pursuit of this promising mAbs market. Sanofi-Aventis is converting a factory near Paris into a biotechnology development hub, with its doors wide open to smaller biotech companies looking to partner on projects. The French pharma giant says that the Rs 1,178 crore ($265 mn) venture marks their commitment to ramping up their work on biologics. It is an opportunity for the company to do partnerships with biotechnology and research companies. The investment of nearly Rs 1,183 crore (€200 mn) will give rise to the first cell culture biotechnology platform of the group to produce mAbs from 2012.
On the other hand, within the same time period, experts are cynical as to whether India can match up to global standards given a the huge amount of investments and the complexity that the process involves. “I do not see that Indian companies achieving much by 2015 and taking their products globally is but a distant dream,” concludes an analyst.
Compared to India, China is a big market for mAbs. Some notable companies include Union Stem Cell and Gene Engineering and Biotech pharmaceutical. In a serious effort towards innovation,China is moving towards areas like stem cells, mAbs, cancer and HIV development and vaccines with investments being pumped in both by the Government and foreign sources. India, experts opine needs exactly the same kind of backing if it need to bolster growth rates of the sector in the region.

monoclonal antibodies (mAb or moAb) are monospecific antibodies that are the same because they are made by identical immune cells that are all clones of a unique parent cell, in contrast to polyclonal antibodies which are made from several different immune cells. Monoclonal antibodies have monovalent affinity, in that they bind to the same epitope.
Given almost any substance, it is possible to produce monoclonal antibodies that specifically bind to that substance; they can then serve to detect or purify that substance. This has become an important tool in biochemistry, molecular biology and medicine. When used as medications, the non-proprietary drug name ends in -mab (see “Nomenclature of monoclonal antibodies“).
Discovery
The idea of a “magic bullet” was first proposed by Paul Ehrlich, who, at the beginning of the 20th century, postulated that, if a compound could be made that selectively targeted against a disease-causing organism, then a toxin for that organism could be delivered along with the agent of selectivity. He and Élie Metchnikoff received the 1908 Nobel Prize for Physiology or Medicine for this work, which led to an effective syphilis treatment by 1910.
In the 1970s, the B-cell cancer multiple myeloma was known, and it was understood that these cancerous B-cells all produce a single type of antibody (a paraprotein). This was used to study the structure of antibodies, but it was not yet possible to produce identical antibodies specific to a given antigen.
Production of monoclonal antibodies involving human–mouse hybrid cells was described by Jerrold Schwaber in 1973[1] and remains widely cited among those using human-derived hybridomas,[2] but claims of priority have been controversial. A science history paper on the subject gave some credit to Schwaber for inventing a technique that was widely cited, but stopped short of suggesting that he had been cheated.[3] The invention was reduced to practice by Cotton and Milstein, and then by Kohler and Milstein. Georges Köhler, César Milstein, and Niels Kaj Jerne in 1975;[4] who shared the Nobel Prize in Physiology or Medicine in 1984 for the discovery. The key idea was to use a line of myeloma cells that had lost their ability to secrete antibodies, come up with a technique to fuse these cells with healthy antibody-producing B-cells, and be able to select for the successfully fused cells. This was put into practice by Milstein and Köhler in their search for a laboratory tool to investigate antibody diversity.[5]
In 1988, Greg Winter and his team pioneered the techniques to humanize monoclonal antibodies,[6] removing the reactions that many monoclonal antibodies caused in some patients.
Production

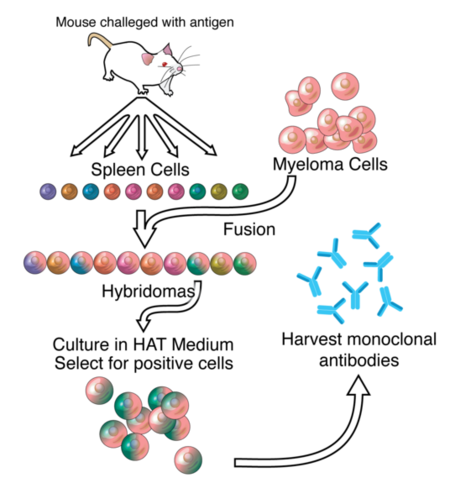
Technician hand-filling wells with a liquid for a research test. This test involves preparation of cultures in which hybrids are grown in large quantities to produce desired antibody. This is effected by fusing myeloma cell and mouse lymphocyte to form a hybrid cell (hybridoma).

Lab technician bathing prepared slides in a solution. This technician prepares slides of monoclonal antibodies for researchers. The cells shown are labeling human breast cancer.
Hybridoma cell production
Monoclonal antibodies are typically made by fusing myeloma cells with the spleen cells from a mouse that has been immunized with the desired antigen. However, recent advances have allowed the use of rabbit B-cells to form a rabbit hybridoma. Polyethylene glycol is used to fuse adjacent plasma membranes, but the success rate is low so a selective medium in which only fused cells can grow is used. This is possible because myeloma cells have lost the ability to synthesize hypoxanthine-guanine-phosphoribosyl transferase (HGPRT), an enzyme necessary for the salvage synthesis of nucleic acids. The absence of HGPRT is not a problem for these cells unless the de novo purine synthesis pathway is also disrupted. By exposing cells to aminopterin (a folic acid analogue, which inhibits dihydrofolate reductase, DHFR), they are unable to use the de novo pathway and become fully auxotrophic for nucleic acids requiring supplementation to survive.
The selective culture medium is called HAT medium because it contains hypoxanthine, aminopterin, and thymidine. This medium is selective for fused (hybridoma) cells. Unfused myeloma cells cannot grow because they lack HGPRT, and thus cannot replicate their DNA. Unfused spleen cells cannot grow indefinitely because of their limited life span. Only fused hybrid cells, referred to as hybridomas, are able to grow indefinitely in the media because the spleen cell partner supplies HGPRT and the myeloma partner has traits that make it immortal (similar to a cancer cell).
This mixture of cells is then diluted and clones are grown from single parent cells on microtitre wells. The antibodies secreted by the different clones are then assayed for their ability to bind to the antigen (with a test such as ELISA or Antigen Microarray Assay) or immuno-dot blot. The most productive and stable clone is then selected for future use.
The hybridomas can be grown indefinitely in a suitable cell culture medium. They can also be injected into mice (in the peritoneal cavity, surrounding the gut). There, they produce tumors secreting an antibody-rich fluid called ascites fluid.
The medium must be enriched during in-vitro selection to further favour hybridoma growth. This can be achieved by the use of a layer of feeder fibrocyte cells or supplement medium such as briclone. Culture-medium conditioned by macrophages can also be used. Production in cell culture is usually preferred as the ascites technique is painful to the animal. Where alternate techniques exist, this method (ascites) is considered unethical.
Purification of monoclonal antibodies
After obtaining either a media sample of cultured hybridomas or a sample of ascites fluid, the desired antibodies must be extracted. The contaminants in the cell culture sample would consist primarily of media components such as growth factors, hormones, and transferrins. In contrast, the in vivo sample is likely to have host antibodies, proteases, nucleases, nucleic acids, and viruses. In both cases, other secretions by the hybridomas such as cytokines may be present. There may also be bacterial contamination and, as a result, endotoxins that are secreted by the bacteria. Depending on the complexity of the media required in cell culture, and thus the contaminants in question, one method (in vivo or in vitro) may be preferable to the other.
The sample is first conditioned, or prepared for purification. Cells, cell debris, lipids, and clotted material are first removed, typically by centrifugation followed by filtration with a 0.45 µm filter. These large particles can cause a phenomenon called membrane fouling in later purification steps. In addition, the concentration of product in the sample may not be sufficient, especially in cases where the desired antibody is one produced by a low-secreting cell line. The sample is therefore condensed by ultrafiltration or dialysis.
Most of the charged impurities are usually anions such as nucleic acids and endotoxins. These are often separated by ion exchange chromatography.[7] Either cation exchange chromatography is used at a low enough pH that the desired antibody binds to the column while anions flow through, or anion exchange chromatography is used at a high enough pH that the desired antibody flows through the column while anions bind to it. Various proteins can also be separated out along with the anions based on their isoelectric point (pI). For example, albumin has a pI of 4.8, which is significantly lower than that of most monoclonal antibodies, which have a pI of 6.1. In other words, at a given pH, the average charge of albumin molecules is likely to be more negative. Transferrin, on the other hand, has a pI of 5.9, so it cannot easily be separated out by this method. A difference in pI of at least 1 is necessary for a good separation.
Transferrin can instead be removed by size exclusion chromatography. The advantage of this purification method is that it is one of the more reliable chromatography techniques. Since we are dealing with proteins, properties such as charge and affinity are not consistent and vary with pH as molecules are protonated and deprotonated, while size stays relatively constant. Nonetheless, it has drawbacks such as low resolution, low capacity and low elution times.
A much quicker, single-step method of separation is Protein A/G affinity chromatography. The antibody selectively binds to Protein A/G, so a high level of purity (generally >80%) is obtained. However, this method may be problematic for antibodies that are easily damaged, as harsh conditions are generally used. A low pH can break the bonds to remove the antibody from the column. In addition to possibly affecting the product, low pH can cause Protein A/G itself to leak off the column and appear in the eluted sample. Gentle elution buffer systems that employ high salt concentrations are also available to avoid exposing sensitive antibodies to low pH. Cost is also an important consideration with this method because immobilized Protein A/G is a more expensive resin.
To achieve maximum purity in a single step, affinity purification can be performed, using the antigen to provide exquisite specificity for the antibody. In this method, the antigen used to generate the antibody is covalently attached to an agarose support. If the antigen is a peptide, it is commonly synthesized with a terminal cysteine, which allows selective attachment to a carrier protein, such as KLH during development and to the support for purification. The antibody-containing media is then incubated with the immobilized antigen, either in batch or as the antibody is passed through a column, where it selectively binds and can be retained while impurities are washed away. An elution with a low pH buffer or a more gentle, high salt elution buffer is then used to recover purified antibody from the support.
To further select for antibodies, the antibodies can be precipitated out using sodium sulfate or ammonium sulfate. Antibodies precipitate at low concentrations of the salt, while most other proteins precipitate at higher concentrations. The appropriate level of salt is added in order to achieve the best separation. Excess salt must then be removed by a desalting method such as dialysis.
The final purity can be analyzed using a chromatogram. Any impurities will produce peaks, and the volume under the peak indicates the amount of the impurity. Alternatively, gel electrophoresis and capillary electrophoresis can be carried out. Impurities will produce bands of varying intensity, depending on how much of the impurity is present.
Antibody heterogeneity
Product heterogeneity is common to monoclonal antibody and other recombinant biological production and is typically introduced either upstream during expression or downstream during manufacturing.
These variants are typically aggregates, deamidation products, glycosylation variants, oxidized amino acid side chains, as well as amino and carboxyl terminal amino acid additions.[8] These seemingly minute changes in a monoclonal antibody’s structure can have a profound effect on preclinical stability and process optimization as well as therapeutic product potency, bioavailability, and immunogenicity. The generally accepted method of purification of process streams for monoclonal antibodies includes capture of the product target with Protein A, elution, acidification to inactivate potential Mammalian viruses, followed by cation exchange chromatography, and finally anion exchange chromatography.
Displacement chromatography has been used to identify and characterize these often unseen variants in quantities that are suitable for subsequent preclinical evaluation regimens such as animal pharmacokinetic studies.[9][10] Knowledge gained during the preclinical development phase is critical for enhanced understanding of product quality and provides a basis for risk management and increased regulatory flexibility. The recent Food and Drug Administration’s Quality by Design initiative attempts to provide guidance on development and to facilitate design of products and processes that maximizes efficacy and safety profile while enhancing product manufacturability.[11]
Recombinant
The production of recombinant monoclonal antibodies involves technologies, referred to as repertoire cloning or phage display/yeast display. Recombinant antibody engineering involves the use of viruses or yeast to create antibodies, rather than mice. These techniques rely on rapid cloning of immunoglobulin gene segments to create libraries of antibodies with slightly different amino acid sequences from which antibodies with desired specificities can be selected.[12] The phage antibody libraries are a variant of the phage antigen libraries first invented by George Pieczenik[13] These techniques can be used to enhance the specificity with which antibodies recognize antigens, their stability in various environmental conditions, their therapeutic efficacy, and their detectability in diagnostic applications.[14] Fermentation chambers have been used to produce these antibodies on a large scale.
Chimeric antibodies
Early on, a major problem for the therapeutic use of monoclonal antibodies in medicine was that initial methods used to produce them yielded mouse, not human antibodies. While structurally similar, differences between the two were sufficient to invoke an immune response when murine monoclonal antibodies were injected into humans, resulting in their rapid removal from the blood, as well as systemic inflammatory effects, and the production of human anti-mouse antibodies (HAMA).[citation needed]
In an effort to overcome this obstacle, approaches using recombinant DNA have been explored since the late 1980s. In one approach, mouse DNA encoding the binding portion of a monoclonal antibody was merged with human antibody-producing DNA in living cells. The expression of this chimeric DNA through cell culture yielded partially mouse, partially human monoclonal antibodies. For this product, the descriptive terms “chimeric” and “humanised” monoclonal antibody have been used to reflect the combination of mouse and human DNA sources used in the recombinant process.[15]
‘Fully’ human monoclonal antibodies
Ever since the discovery that monoclonal antibodies could be generated, scientists have targeted the creation of ‘fully’ human antibodies to avoid some of the side effects of humanised or chimeric antibodies. Two successful approaches have been identified: transgenic mice[16] and phage display.
Transgenic mice technology is by far the most successful approach to making ‘fully’ human monoclonal antibody therapeutics: 7 of the 9 ‘fully’ human monoclonal antibody therapeutics on the market were derived in this manner.[17]
Transgenic mice have been exploited by a number of commercial organisations:
- Medarex — who marketed their UltiMab platform. Medarex were acquired in July 2009 by Bristol Myers Squibb[18]
- Abgenix — who marketed their Xenomouse technology. Abgenix were acquired in April 2006 by Amgen.[19]
- Regeneron‘s VelocImmune technology.[20]
- Kymab – who market their Kymouse technology.[21]
One of the most successful commercial organisations using phage display technology was Cambridge Antibody Technology (CAT). Scientists at CAT demonstrated that phage display could be used such that variable antibody domains could be expressed on filamentous phage antibodies. This was reported in a key Nature publication.[22]
Other significant publications include:
- Marks JD, Hoogenboom HR, Bonnert TP, McCafferty J, Griffiths AD, Winter G (December 1991). “By-passing immunization. Human antibodies from V-gene libraries displayed on phage”. J. Mol. Biol. 222 (3): 581–597. doi:10.1016/0022-2836(91)90498-U. PMID 1748994.
- Carmen S, Jermutus L (July 2002). “Concepts in antibody phage display”. Brief Funct Genomic Proteomic 1 (2): 189–203. doi:10.1093/bfgp/1.2.189. PMID 15239904.
CAT developed their display technologies further into several, patented antibody discovery/functional genomics tools, which were named ProximolTM[23] and ProAbTM. ProAb was announced in December 1997[24] and involved high throughput screening of antibody libraries against diseased and non-diseased tissue, whilst Proximol used a free radical enzymatic reaction to label molecules in proximity to a given protein.[25][26]
Monoclonal antibodies have been generated and approved to treat cancer, cardiovascular disease, inflammatory diseases, macular degeneration, transplant rejection, multiple sclerosis, and viral infection (see monoclonal antibody therapy).
In August 2006 the Pharmaceutical Research and Manufacturers of America reported that U.S. companies had 160 different monoclonal antibodies in clinical trials or awaiting approval by the Food and Drug Administration.[27]
Applications
Diagnostic tests
Once monoclonal antibodies for a given substance have been produced, they can be used to detect the presence of this substance. The Western blot test and immuno dot blot tests detect the protein on a membrane. They are also very useful in immunohistochemistry, which detect antigen in fixed tissue sections and immunofluorescence test, which detect the substance in a frozen tissue section or in live cells.
Therapeutic treatment
Cancer treatment
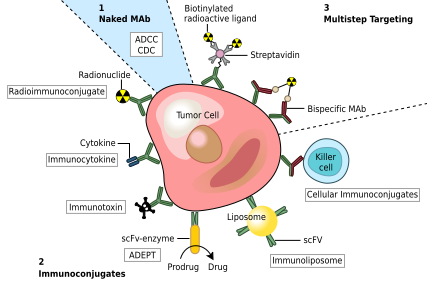
One possible treatment for cancer involves monoclonal antibodies that bind only to cancer cell-specific antigens and induce an immunological response against the target cancer cell. Such mAb could also be modified for delivery of a toxin, radioisotope, cytokine or other active conjugate; it is also possible to design bispecific antibodies that can bind with their Fab regions both to target antigen and to a conjugate or effector cell. In fact, every intact antibody can bind to cell receptors or other proteins with its Fc region.
The illustration below shows all these possibilities:
MAbs approved by the FDA include[29]
Autoimmune diseases
Monoclonal antibodies used for autoimmune diseases include infliximab and adalimumab, which are effective in rheumatoid arthritis, Crohn’s disease and ulcerative Colitis by their ability to bind to and inhibit TNF-α.[30] Basiliximab and daclizumab inhibit IL-2 on activated T cells and thereby help prevent acute rejection of kidney transplants.[30] Omalizumab inhibits human immunoglobulin E (IgE) and is useful in moderate-to-severe allergic asthma.
Examples
Below are examples of clinically important monoclonal antibodies.
| Main category | Type | Application | Mechanism/Target | Mode |
|---|---|---|---|---|
| Anti- inflammatory |
infliximab[30] | inhibits TNF-α | chimeric | |
| adalimumab | inhibits TNF-α | human | ||
| basiliximab[30] |
|
inhibits IL-2 on activated T cells | chimeric | |
| daclizumab[30] |
|
inhibits IL-2 on activated T cells | humanized | |
| omalizumab |
|
inhibits human immunoglobulin E (IgE) | humanized | |
| Anti-cancer | gemtuzumab[30] |
|
targets myeloid cell surface antigen CD33 on leukemia cells | humanized |
| alemtuzumab[30] | targets an antigen CD52 on T- and B-lymphocytes | humanized | ||
| rituximab[30] | targets phosphoprotein CD20 on B lymphocytes | chimeric | ||
| trastuzumab |
|
targets the HER2/neu (erbB2) receptor | humanized | |
| nimotuzumab |
|
EGFR inhibitor | Humanized | |
| cetuximab |
|
EGFR inhibitor | Chimeric | |
| bevacizumab |
|
inhibits VEGF | humanized | |
| Other | palivizumab[30] |
|
inhibits an RSV fusion (F) protein | humanized |
| abciximab[30] |
|
inhibits the receptor GpIIb/IIIa on platelets | chimeric |
See also
- Antibody mimetic
- Immunotoxins, which sometimes use monoclonal antibodies as the targeting mechanism
- List of monoclonal antibodies
- Monoclonal antibody therapy
- Nomenclature of monoclonal antibodies
- Polyclonal antibodies
- Queen Mab Small mythological figure symbolizing hope (popular culture, used as biotech pun).
References
- Schwaber, J.; Cohen, E. P. (1973). “Human x mouse somatic cell hybrid clone secreting immunoglobulins of both parental types”. Nature 244 (5416): 444–447. Bibcode:1973Natur.244..444S. doi:10.1038/244444a0. PMID 4200460. edit
- Jump up ^ Science Citation Index
- Jump up ^ Cambrosio, A.; Keating, P. (1992). “Between fact and technique: the beginnings of hybridoma technology”. Journal of the History of Biology 25 (2): 175–230. doi:10.1007/BF00162840. PMID 11623041. edit
- Jump up ^ Köhler, G.; Milstein, C. (1975). “Continuous cultures of fused cells secreting antibody of predefined specificity”. Nature 256 (5517): 495–497. Bibcode:1975Natur.256..495K. doi:10.1038/256495a0. PMID 1172191. edit
- Jump up ^ The Story of César Milstein and Monoclonal Antibodies.
- Jump up ^ Riechmann L, Clark M, Waldmann H, Winter G (March 1988). “Reshaping human antibodies for therapy”. Nature 332 (6162): 323–327. Bibcode:1988Natur.332..323R. doi:10.1038/332323a0. PMID 3127726.
- Jump up ^ Vlasek J, Ionescu R (2008). “Hetergeneity of Monoclonal Antibodies Revealed by Charge-Sensitive Methods”. Current Pharmaceutical Biotechnology 9 (6): 468–481. doi:10.2174/138920108786786402. PMID 19075686.
- Jump up ^ Beck A, Wurch T, Bailly C, Corvaia N (May 2010). “Strategies and challenges for the next generation of therapeutic antibodies”. Nat. Rev. Immunol. 10 (5): 345–52. doi:10.1038/nri2747. PMID 20414207.
- Jump up ^ Khawli LA, Goswami S, Hutchinson R, et al. (2010). “Charge variants in IgG1: Isolation, characterization, in vitro binding properties and pharmacokinetics in rats”. MAbs 2 (6): 613–24. doi:10.4161/mabs.2.6.13333. PMC 3011216. PMID 20818176.
- Jump up ^ Zhang T, Bourret J, Cano T (August 2011). “Isolation and characterization of therapeutic antibody charge variants using cation exchange displacement chromatography”. J Chromatogr A 1218 (31): 5079–86. doi:10.1016/j.chroma.2011.05.061. PMID 21700290.
- Jump up ^ Rathore AS, Winkle H (January 2009). “Quality by design for biopharmaceuticals”. Nat. Biotechnol. 27 (1): 26–34. doi:10.1038/nbt0109-26. PMID 19131992.
- Jump up ^ Siegel DL (2002). “Recombinant monoclonal antibody technology”. Transfusion clinique et biologique : journal de la Société française de transfusion sanguine 9 (1): 15–22. doi:10.1016/S1246-7820(01)00210-5. PMID 11889896.
- Jump up ^ “Dr. George Pieczenik”. LMB Alumni. MRC Laboratory of Molecular Biology (LMB). 17 September 2009.
- Jump up ^ Schmitz U, Versmold A, Kaufmann P, Frank HG (2000). “Phage display: a molecular tool for the generation of antibodies—a review”. Placenta 21 (Suppl A): S106–S112. doi:10.1053/plac.1999.0511. PMID 10831134.
- Jump up ^ Chadd HE, Chamow SM (April 2001). “Therapeutic antibody expression technology”. Curr. Opin. Biotechnol. 12 (2): 188–94. doi:10.1016/S0958-1669(00)00198-1. PMID 11287236.
- Jump up ^ Lonberg N, Huszar D (1995). “Human antibodies from transgenic mice”. Int. Rev. Immunol. 13 (1): 65–93. doi:10.3109/08830189509061738. PMID 7494109.
- Jump up ^ http://en.wikipedia.org/wiki/Monoclonal_antibody_therapy
- Jump up ^ “Bristol-Myers Buys Medarex Drugmaker for $2.4 Billion (Update3)”.
- Jump up ^ “Amgen Completes Acquisition of Abgenix; Acquisition Provides Amgen with Full Ownership of Panitumumab and Eliminates a Denosumab Royalty”.
- Jump up ^ http://www.regeneron.com/velocimmune.html
- Jump up ^ “Proprietary antibody platform”.
- Jump up ^ McCafferty, J.; Griffiths, A.; Winter, G.; Chiswell, D. (1990). “Phage antibodies: filamentous phage displaying antibody variable domains”. Nature 348 (6301): 552–554. Bibcode:1990Natur.348..552M. doi:10.1038/348552a0. PMID 2247164. edit
- Jump up ^ Osbourn JK (2002). “Proximity-guided (ProxiMol) antibody selection”. Methods Mol. Biol. 178: 201–5. PMID 11968489.
- Jump up ^ “Cambridge Antibody Technology”.
- Jump up ^ Osbourn JK, Derbyshire EJ, Vaughan TJ, Field AW, Johnson KS (January 1998). “Pathfinder selection: in situ isolation of novel antibodies”. Immunotechnology 3 (4): 293–302. doi:10.1016/S1380-2933(97)10007-0. PMID 9530562.
- Jump up ^ “The Current State of Proteomic Technology”.
- Jump up ^ PhRMA Reports Identifies More than 400 Biotech Drugs in Development. Pharmaceutical Technology, August 24, 2006. Retrieved 2006-09-04.
- Jump up ^ Modified from Carter P (November 2001). “Improving the efficacy of antibody-based cancer therapies”. Nat. Rev. Cancer 1 (2): 118–29. doi:10.1038/35101072. PMID 11905803.
- Jump up ^ Takimoto CH, Calvo E. “Principles of Oncologic Pharmacotherapy” in Pazdur R, Wagman LD, Camphausen KA, Hoskins WJ (Eds) Cancer Management
- ^ Jump up to: a b c d e f g h i j Rang, H. P. (2003). Pharmacology. Edinburgh: Churchill Livingstone. pp. 241, for the examples infliximab, basiliximab, abciximab, daclizumab, palivusamab, gemtuzumab, alemtuzumab and rituximab, and mechanism and mode. ISBN 0-443-07145-4.
reference 24: should be
chapter 34. Therapeutic Antibodies and Immunologic Conjugates. In Clinical Oncology, 4th Edition, edited by Abeloff MD, Armitage JO, Niederhuber JE, Kastan MB and McKenna G. Publisher Elsevier Inc. 2008.
External links
| Library resources |
|---|
| About Monoclonal antibody |
- Monoclonal Antibodies Animation (Flash Required)
- Monoclonal Antibodies, from John W. Kimball’s online biology textbook
- Monoclonal antibodies at the US National Library of Medicine Medical Subject Headings (MeSH)
- Antibodypedia, open-access virtual repository publishing data and commentary on any antibodies available to the scientific community.
list of monoclonal antibodies
http://en.wikipedia.org/wiki/List_of_monoclonal_antibodies
This list includes approved and investigational drugs as well as drugs that have been withdrawn from market; consequently, the column Use does not necessarily indicate clinical usage.
| Name | Trade name | Type | Source | Target | Use |
|---|---|---|---|---|---|
| 3F8 | mab | mouse | GD2 | neuroblastoma | |
| 8H9[1] | mab | mouse | B7-H3 | neuroblastoma, sarcoma, metastatic brain cancers | |
| Abagovomab[2] | mab | mouse | CA-125 (imitation) | ovarian cancer | |
| Abciximab | ReoPro | Fab | chimeric | CD41 (integrin alpha-IIb) | platelet aggregation inhibitor |
| Actoxumab[3] | mab | human | Clostridium difficile | Clostridium difficile infection | |
| Adalimumab | Humira | mab | human | TNF-α | Rheumatoid arthritis, Crohn’s Disease, Plaque Psoriasis, Psoriatic Arthritis, Ankylosing Spondylitis, Juvenile Idiopathic Arthritis |
| Adecatumumab[4] | mab | human | EpCAM | prostate and breast cancer | |
| Afelimomab | F(ab’)2 | mouse | TNF-α | sepsis | |
| Afutuzumab[5] | mab | humanized | CD20 | lymphoma | |
| Alacizumab pegol[6] | F(ab’)2 | humanized | VEGFR2 | cancer | |
| ALD518[7] | ? | humanized | IL-6 | rheumatoid arthritis | |
| Alemtuzumab[8] | Campath, MabCampath | mab | humanized | CD52 | CLL, CTCL |
| Alirocumab[9] | mab | human | NARP-1 | hypercholesterolemia | |
| Altumomab pentetate | Hybri-ceaker | mab | mouse | CEA | colorectal cancer (diagnosis) |
| Amatuximab[10] | mab | chimeric | mesothelin | cancer | |
| Anatumomab mafenatox | Fab | mouse | TAG-72 | non-small cell lung carcinoma | |
| Anifrolumab[11] | mab | human | interferon α/β receptor | systemic lupus erythematosus | |
| Anrukinzumab[6] (= IMA-638)[12] | mab | humanized | IL-13 | ? | |
| Apolizumab[13] | mab | humanized | HLA-DR ? | hematological cancers | |
| Arcitumomab | CEA-Scan | Fab’ | mouse | CEA | gastrointestinal cancers (diagnosis) |
| Aselizumab[14] | mab | humanized | L-selectin (CD62L) | severely injured patients | |
| Atinumab[15] | mab | human | RTN4 | ? | |
| Atlizumab (= tocilizumab) | Actemra, RoActemra | mab | humanized | IL-6 receptor | rheumatoid arthritis |
| Atorolimumab | mab | human | Rhesus factor | hemolytic disease of the newborn[citation needed] | |
| Bapineuzumab[16] | mab | humanized | beta amyloid | Alzheimer’s disease | |
| Basiliximab | Simulect | mab | chimeric | CD25 (α chain of IL-2 receptor) | prevention of organ transplant rejections |
| Bavituximab[2] | mab | chimeric | phosphatidylserine | cancer, viral infections | |
| Bectumomab | LymphoScan | Fab’ | mouse | CD22 | non-Hodgkin’s lymphoma (detection) |
| Belimumab | Benlysta, LymphoStat-B | mab | human | BAFF | non-Hodgkin lymphoma etc. |
| Benralizumab | mab | humanized | CD125 | asthma | |
| Bertilimumab[14] | mab | human | CCL11 (eotaxin-1) | severe allergic disorders | |
| Besilesomab[17] | Scintimun | mab | mouse | CEA-related antigen | inflammatory lesions and metastases (detection) |
| Bevacizumab[8] | Avastin | mab | humanized | VEGF-A | metastatic cancer |
| Bezlotoxumab[18] | mab | human | Clostridium difficile | Clostridium difficile infection | |
| Biciromab | FibriScint | Fab’ | mouse | fibrin II, beta chain | thromboembolism (diagnosis) |
| Bimagrumab[19] | mab | human | ACVR2B | myostatin inhibitor | |
| Bivatuzumab mertansine | mab | humanized | CD44 v6 | squamous cell carcinoma | |
| Blinatumomab | BiTE | mouse | CD19 | cancer | |
| Blosozumab[20] | mab | humanized | SOST | osteoporosis | |
| Brentuximab vedotin[21] | mab | chimeric | CD30 (TNFRSF8) | hematologic cancers | |
| Briakinumab[22] | mab | human | IL-12, IL-23 | psoriasis, rheumatoid arthritis, inflammatory bowel diseases, multiple sclerosis | |
| Brodalumab[23] | mab | human | IL-17 | inflammatory diseases | |
| Canakinumab[24] | Ilaris | mab | human | IL-1? | rheumatoid arthritis |
| Cantuzumab mertansine | mab | humanized | mucin CanAg | colorectal cancer etc. | |
| Cantuzumab ravtansine[20] | mab | humanized | MUC1 | cancers | |
| Caplacizumab[25] | mab | humanized | VWF | ? | |
| Capromab pendetide | Prostascint | mab | mouse | prostatic carcinoma cells | prostate cancer (detection) |
| Carlumab[26] | mab | human | MCP-1 | oncology/immune indications | |
| Catumaxomab[16] | Removab | 3funct | rat/mouse hybrid | EpCAM, CD3 | ovarian cancer, malignant ascites, gastric cancer |
| CC49 | mab | mouse | TAG-72 | tumor detection | |
| Cedelizumab | mab | humanized | CD4 | prevention of organ transplant rejections, treatment of autoimmune diseases | |
| Certolizumab pegol[4] | Cimzia | Fab’ | humanized | TNF-α | Crohn’s disease |
| Cetuximab | Erbitux | mab | chimeric | EGFR | metastatic colorectal cancer and head and neck cancer |
| Ch.14.18 [1] | mab | chimeric | ??? | neuroblastoma | |
| Citatuzumab bogatox[5] | Fab | humanized | EpCAM | ovarian cancer and other solid tumors | |
| Cixutumumab | mab | human | IGF-1 receptor | solid tumors | |
| Clazakizumab[27] | mab | humanized | Oryctolagus cuniculus | rheumatoid arthritis | |
| Clenoliximab | mab | chimeric | CD4 | rheumatoid arthritis | |
| Clivatuzumab tetraxetan[28] | hPAM4-Cide | mab | humanized | MUC1 | pancreatic cancer |
| Conatumumab[5] | mab | human | TRAIL-R2 | cancer | |
| Concizumab[19] | mab | humanized | TFPI | bleeding | |
| Crenezumab[29] | mab | humanized | 1-40-β-amyloid | Alzheimer’s disease | |
| CR6261 | mab | human | Influenza A hemagglutinin | infectious disease/influenza A | |
| Dacetuzumab[6] | mab | humanized | CD40 | hematologic cancers | |
| Daclizumab | Zenapax | mab | humanized | CD25 (α chain of IL-2 receptor) | prevention of organ transplant rejections |
| Dalotuzumab[30] | mab | humanized | insulin-like growth factor I receptor | cancer etc. | |
| Daratumumab[31] | mab | human | CD38 (cyclic ADP ribose hydrolase) | ? | |
| Demcizumab[32] | mab | humanized | DLL4 | cancer | |
| Denosumab[33] | Prolia | mab | human | RANKL | osteoporosis, bone metastases etc. |
| Detumomab | mab | mouse | B-lymphoma cell | lymphoma | |
| Dorlimomab aritox[34] | F(ab’)2 | mouse | ? | ? | |
| Drozitumab[35] | mab | human | DR5 | cancer etc. | |
| Duligotumab[36] | mab | human | HER3 | ? | |
| Dupilumab[37] | mab | human | IL4 | atopic diseases | |
| Dusigitumab[38] | mab | human | ILGF2 | cancer | |
| Ecromeximab[13] | mab | chimeric | GD3 ganglioside | malignant melanoma | |
| Eculizumab[13] | Soliris | mab | humanized | C5 | paroxysmal nocturnal hemoglobinuria |
| Edobacomab | mab | mouse | endotoxin | sepsis caused by Gram-negative bacteria | |
| Edrecolomab | Panorex | mab | mouse | EpCAM | colorectal carcinoma |
| Efalizumab[39] | Raptiva | mab | humanized | LFA-1 (CD11a) | psoriasis (blocks T-cell migration) |
| Efungumab[2] | Mycograb | scFv | human | Hsp90 | invasive Candida infection |
| Eldelumab[40] | mab | human | interferon gamma-induced protein | Crohn’s disease, ulcerative colitis | |
| Elotuzumab | mab | humanized | SLAMF7 | multiple myeloma | |
| Elsilimomab | mab | mouse | IL-6 | ? | |
| Enavatuzumab[41] | mab | humanized | TWEAK receptor | cancer etc. | |
| Enlimomab pegol[42] | mab | mouse | ICAM-1 (CD54) | ? | |
| Enokizumab[43] | mab | humanized | IL9 | asthma | |
| Enoticumab[36] | mab | human | DLL4 | ? | |
| Ensituximab[44] | mab | chimeric | 5AC | cancer | |
| Epitumomab cituxetan[45] | mab | mouse | episialin | ? | |
| Epratuzumab | mab | humanized | CD22 | cancer, SLE | |
| Erlizumab[46] | F(ab’)2 | humanized | ITGB2 (CD18) | heart attack, stroke, traumatic shock | |
| Ertumaxomab[16] | Rexomun | 3funct | rat/mouse hybrid | HER2/neu, CD3 | breast cancer etc. |
| Etaracizumab | Abegrin | mab | humanized | integrin αvβ3 | melanoma, prostate cancer, ovarian cancer etc. |
| Etrolizumab[47] | mab | humanized | integrin α7 β7 | inflammatory bowel disease | |
| Evolocumab[19] | mab | human | PCSK9 | hypocholesterolemia | |
| Exbivirumab[48] | mab | human | hepatitis B surface antigen | hepatitis B | |
| Fanolesomab[49] | NeutroSpec | mab | mouse | CD15 | appendicitis (diagnosis) |
| Faralimomab | mab | mouse | interferon receptor | ? | |
| Farletuzumab | mab | humanized | folate receptor 1 | ovarian cancer | |
| Fasinumab[50] | mab | human | HNGF | ? | |
| FBTA05[51][52] | Lymphomun | 3funct | rat/mouse hybrid | CD20 | chronic lymphocytic leukaemia |
| Felvizumab | mab | humanized | respiratory syncytial virus | respiratory syncytial virus infection | |
| Fezakinumab[53][54][55] | mab | human | IL-22 | rheumatoid arthritis, psoriasis | |
| Ficlatuzumab[56] | mab | humanized | HGF | cancer etc. | |
| Figitumumab | mab | human | IGF-1 receptor | adrenocortical carcinoma, non-small cell lung carcinoma etc. | |
| Flanvotumab[57] | mab | human | glycoprotein 75 | melanoma | |
| Fontolizumab[13] | HuZAF | mab | humanized | IFN-γ | Crohn’s disease etc. |
| Foralumab[58] | mab | human | CD3 epsilon | ? | |
| Foravirumab[5] | mab | human | rabies virus glycoprotein | rabies (prophylaxis) | |
| Fresolimumab[59] | mab | human | TGF-β | idiopathic pulmonary fibrosis, focal segmental glomerulosclerosis, cancer | |
| Fulranumab[60] | mab | human | NGF | pain | |
| Futuximab[36] | mab | chimeric | EGFR | ? | |
| Galiximab | mab | chimeric | CD80 | B-cell lymphoma | |
| Ganitumab[61] | mab | human | IGF-I | cancer | |
| Gantenerumab[24] | mab | human | beta amyloid | Alzheimer’s disease | |
| Gavilimomab[46] | mab | mouse | CD147 (basigin) | graft versus host disease | |
| Gemtuzumab ozogamicin | Mylotarg | mab | humanized | CD33 | acute myelogenous leukemia |
| Gevokizumab[62] | mab | humanized | IL-1β | diabetes etc. | |
| Girentuximab[31] | Rencarex | mab | chimeric | carbonic anhydrase 9 (CA-IX) | clear cell renal cell carcinoma[63] |
| Glembatumumab vedotin[64][65] | mab | human | GPNMB | melanoma, breast cancer | |
| Golimumab[48] | Simponi | mab | human | TNF-α | rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis |
| Gomiliximab | mab | chimeric | CD23 (IgE receptor) | allergic asthma | |
| GS6624 [66] | mab | ? | ? | idiopathic pulmonary fibrosis and solid tumors | |
| Guselkumab[67] | mab | human | IL13 | psoriasis | |
| Ibalizumab[24] | mab | humanized | CD4 | HIV infection | |
| Ibritumomab tiuxetan | Zevalin | mab | mouse | CD20 | non-Hodgkin’s lymphoma |
| Icrucumab[68] | mab | human | VEGFR-1 | cancer etc. | |
| Igovomab | Indimacis-125 | F(ab’)2 | mouse | CA-125 | ovarian cancer (diagnosis) |
| Imciromab | Myoscint | mab | mouse | cardiac myosin | cardiac imaging |
| Imgatuzumab[36] | mab | humanized | EGFR | cancer | |
| Inclacumab[25] | mab | human | selectin P | ? | |
| Indatuximab ravtansine[20] | mab | chimeric | SDC1 | cancer | |
| Infliximab | Remicade | mab | chimeric | TNF-α | rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis, psoriasis, Crohn’s disease, ulcerative colitis |
| Intetumumab[69][70][71] | mab | human | CD51 | solid tumors (prostate cancer, melanoma) | |
| Inolimomab | mab | mouse | CD25 (α chain of IL-2 receptor) | graft versus host disease | |
| Inotuzumab ozogamicin[17] | mab | humanized | CD22 | cancer | |
| Ipilimumab[33] | Yervoy | mab | human | CD152 | melanoma |
| Iratumumab[33] | mab | human | CD30 (TNFRSF8) | Hodgkin’s lymphoma | |
| Itolizumab[58] | mab | humanized | CD6 | ? | |
| Ixekizumab[72] | mab | humanized | IL-17A | autoimmune diseases | |
| Keliximab | mab | chimeric | CD4 | chronic asthma | |
| Labetuzumab[39] | CEA-Cide | mab | humanized | CEA | colorectal cancer |
| Lambrolizumab[73] | mab | humanized | PDCD1 | antineoplastic agent | |
| Lampalizumab[36] | mab | humanized | CFD | ? | |
| Lebrikizumab[74] | mab | humanized | IL-13 | asthma | |
| Lemalesomab[46] | mab | mouse | NCA-90 (granulocyte antigen) | diagnostic agent | |
| Lerdelimumab[8] | mab | human | TGF beta 2 | reduction of scarring after glaucoma surgery | |
| Lexatumumab[2] | mab | human | TRAIL-R2 | cancer | |
| Libivirumab[48] | mab | human | hepatitis B surface antigen | hepatitis B | |
| Ligelizumab[36] | mab | humanized | IGHE | ? | |
| Lintuzumab | mab | humanized | CD33 | cancer | |
| Lirilumab[36] | mab | human | KIR2D | ? | |
| Lodelcizumab[19] | mab | humanized | PCSK9 | hypercholesterolemia | |
| Lorvotuzumab mertansine | mab | humanized | CD56 | cancer | |
| Lucatumumab[6] | mab | human | CD40 | multiple myeloma, non-Hodgkin’s lymphoma, Hodgkin’s lymphoma | |
| Lumiliximab[4] | mab | chimeric | CD23 (IgE receptor) | chronic lymphocytic leukemia | |
| Mapatumumab[16] | mab | human | TRAIL-R1 | cancer | |
| Margetuximab[75] | mab | humanized | ch4D5 | cancer | |
| Maslimomab | ? | mouse | T-cell receptor | ? | |
| Mavrilimumab[76] | mab | human | GMCSF receptor α-chain | rheumatoid arthritis | |
| Matuzumab[14] | mab | humanized | EGFR | colorectal, lung and stomach cancer | |
| Mepolizumab | Bosatria | mab | humanized | IL-5 | asthma and white blood cell diseases |
| Metelimumab[49] | mab | human | TGF beta 1 | systemic scleroderma | |
| Milatuzumab[6] | mab | humanized | CD74 | multiple myeloma and other hematological malignancies | |
| Minretumomab | mab | mouse | TAG-72 | ? | |
| Mitumomab | mab | mouse | GD3 ganglioside | small cell lung carcinoma | |
| Mogamulizumab[77] | mab | humanized | CCR4 | cancer | |
| Morolimumab | mab | human | Rhesus factor | ? | |
| Motavizumab[2] | Numax | mab | humanized | respiratory syncytial virus | respiratory syncytial virus (prevention) |
| Moxetumomab pasudotox[78] | mab | mouse | CD22 | cancer | |
| Muromonab-CD3 | Orthoclone OKT3 | mab | mouse | CD3 | prevention of organ transplant rejections |
| Nacolomab tafenatox | Fab | mouse | C242 antigen | colorectal cancer | |
| Namilumab[15] | mab | human | CSF2 | ? | |
| Naptumomab estafenatox[79] | Fab | mouse | 5T4 | non-small cell lung carcinoma, renal cell carcinoma | |
| Narnatumab[80] | mab | human | RON | cancer | |
| Natalizumab | Tysabri | mab | humanized | integrin α4 | multiple sclerosis, Crohn’s disease |
| Nebacumab | mab | human | endotoxin | sepsis | |
| Necitumumab[81] | mab | human | EGFR | non-small cell lung carcinoma | |
| Nerelimomab | mab | mouse | TNF-α | ? | |
| Nesvacumab[82] | mab | human | angiopoietin 2 | cancer | |
| Nimotuzumab[33][83] | Theracim, Theraloc | mab | humanized | EGFR | squamous cell carcinoma, head and neck cancer, nasopharyngeal cancer, glioma |
| Nivolumab[84] | mab | human | IgG4 | cancer | |
| Nofetumomab merpentan | Verluma | Fab | mouse | ? | cancer (diagnosis) |
| Ocaratuzumab[85] | mab | humanized | CD20 | cancer | |
| Ocrelizumab[33] | mab | humanized | CD20 | rheumatoid arthritis, lupus erythematosus etc. | |
| Odulimomab | mab | mouse | LFA-1 (CD11a) | prevention of organ transplant rejections, immunological diseases | |
| Ofatumumab[16] | Arzerra | mab | human | CD20 | chronic lymphocytic leukemia etc. |
| Olaratumab | mab | human | PDGF-R α | cancer | |
| Olokizumab[58] | mab | humanized | IL6 | ? | |
| Omalizumab[46] | Xolair | mab | humanized | IgE Fc region | allergic asthma |
| Onartuzumab[86] | mab | humanized | human scatter factor receptor kinase | cancer | |
| Ontuxizumab[87] | mab | chimeric/humanized | TEM1 | cancer | |
| Oportuzumab monatox[81] | scFv | humanized | EpCAM | cancer | |
| Oregovomab[49] | OvaRex | mab | mouse | CA-125 | ovarian cancer |
| Orticumab[36] | mab | human | oxLDL | ? | |
| Otelixizumab[6] | mab | chimeric/humanized | CD3 | diabetes mellitus type 1 | |
| Oxelumab[88] | mab | human | OX-40 | asthma | |
| Ozanezumab[89] | mab | humanized | NOGO-A | ALS and multiple sclerosis | |
| Ozoralizumab[90] | mab | humanized | TNF-α | inflammation | |
| Pagibaximab[16] | mab | chimeric | lipoteichoic acid | sepsis (Staphylococcus) | |
| Palivizumab | Synagis, Abbosynagis | mab | humanized | F protein of respiratory syncytial virus | respiratory syncytial virus (prevention) |
| Panitumumab[48] | Vectibix | mab | human | EGFR | colorectal cancer |
| Panobacumab[81] | mab | human | Pseudomonas aeruginosa | Pseudomonas aeruginosa infection | |
| Parsatuzumab[36] | mab | human | EGFL7 | cancer | |
| Pascolizumab[13] | mab | humanized | IL-4 | asthma | |
| Pateclizumab[20] | mab | humanized | LTA | TNF | |
| Patritumab[25] | mab | human | HER3 | cancer | |
| Pemtumomab | Theragyn | ? | mouse | MUC1 | cancer |
| Perakizumab[36] | mab | humanized | IL17A | arthritis | |
| Pertuzumab | Omnitarg | mab | humanized | HER2/neu | cancer |
| Pexelizumab[39] | scFv | humanized | C5 | reduction of side effects of cardiac surgery | |
| Pidilizumab[91] | mab | humanized | PD-1 | cancer and infectious diseases | |
| Pinatuzumab vedotin[19] | mab | humanized | CD22 | cancer | |
| Pintumomab | mab | mouse | adenocarcinoma antigen | adenocarcinoma (imaging) | |
| Placulumab[92] | mab | human | human TNF | ? | |
| Polatuzumab vedotin[19] | mab | humanized | CD79B | ? | |
| Ponezumab[93] | mab | humanized | human beta-amyloid | Alzheimer’s disease | |
| Priliximab | mab | chimeric | CD4 | Crohn’s disease, multiple sclerosis | |
| Pritoxaximab[19] | mab | chimeric | E. coli shiga toxin type-1 | ? | |
| Pritumumab | mab | human | vimentin | brain cancer | |
| PRO 140 | ? | humanized | CCR5 | HIV infection | |
| Quilizumab[25] | mab | humanized | IGHE | ? | |
| Racotumomab[81] | mab | mouse | N–glycolylneuraminic acid | cancer | |
| Radretumab[15] | mab | human | fibronectin extra domain-B | cancer | |
| Rafivirumab[5] | mab | human | rabies virus glycoprotein | rabies (prophylaxis) | |
| Ramucirumab | mab | human | VEGFR2 | solid tumors | |
| Ranibizumab[4] | Lucentis | Fab | humanized | VEGF-A | macular degeneration (wet form) |
| Raxibacumab[17] | mab | human | anthrax toxin, protective antigen | anthrax (prophylaxis and treatment) | |
| Regavirumab | mab | human | cytomegalovirus glycoprotein B | cytomegalovirus infection | |
| Reslizumab[39] | mab | humanized | IL-5 | inflammations of the airways, skin and gastrointestinal tract | |
| Rilotumumab[94] | mab | human | HGF | solid tumors | |
| Rituximab | MabThera, Rituxan | mab | chimeric | CD20 | lymphomas, leukemias, some autoimmune disorders |
| Robatumumab | mab | human | IGF-1 receptor | cancer | |
| Roledumab[58] | mab | human | RHD | ? | |
| Romosozumab[95] | mab | humanized | scleroscin | osteoporosis | |
| Rontalizumab[96] | mab | humanized | IFN-α | systemic lupus erythematosus | |
| Rovelizumab | LeukArrest | mab | humanized | CD11, CD18 | haemorrhagic shock etc. |
| Ruplizumab[8] | Antova | mab | humanized | CD154 (CD40L) | rheumatic diseases |
| Samalizumab[97] | mab | humanized | CD200 | cancer | |
| Sarilumab[98] | mab | human | IL6 | rheumatoid arthritis, ankylosing spondylitis | |
| Satumomab pendetide | mab | mouse | TAG-72 | cancer (diagnosis) | |
| Secukinumab[99] | mab | human | IL-17A | uveitis, rheumatoid arthritis psoriasis | |
| Seribantumab[19] | mab | human | ERBB3 | cancer | |
| Setoxaximab[19] | mab | chimeric | E. coli shiga toxin type-1 | ? | |
| Sevirumab | ? | human | cytomegalovirus | cytomegalovirus infection | |
| Sibrotuzumab | mab | humanized | FAP | cancer | |
| Sifalimumab[100] | mab | humanized | IFN-α | SLE, dermatomyositis, polymyositis | |
| Siltuximab | mab | chimeric | IL-6 | cancer | |
| Simtuzumab[36] | mab | humanized | LOXL2 | ? | |
| Siplizumab[13] | mab | humanized | CD2 | psoriasis, graft-versus-host disease (prevention) | |
| Sirukumab[101] | mab | human | IL-6 | rheumatoid arthritis | |
| Solanezumab[81] | mab | humanized | beta amyloid | Alzheimer’s disease | |
| Solitomab[25] | mab | mouse | EpCAM | ? | |
| Sonepcizumab[102] | ? | humanized | sphingosine-1-phosphate | choroidal and retinal neovascularization | |
| Sontuzumab[83] | mab | humanized | episialin | ? | |
| Stamulumab[33][83] | mab | human | myostatin | muscular dystrophy | |
| Sulesomab | LeukoScan | Fab’ | mouse | NCA-90 (granulocyte antigen) | osteomyelitis (imaging) |
| Suvizumab[64] | mab | humanized | HIV-1 | viral infections | |
| Tabalumab[103] | mab | human | BAFF | B-cell cancers | |
| Tacatuzumab tetraxetan | AFP-Cide | mab | humanized | alpha-fetoprotein | cancer |
| Tadocizumab[83] | Fab | humanized | integrin αIIbβ3 | percutaneous coronary intervention | |
| Talizumab | mab | humanized | IgE | allergic reaction | |
| Tanezumab[5] | mab | humanized | NGF | pain | |
| Taplitumomab paptox[46] | mab | mouse | CD19 | cancer[citation needed] | |
| Tefibazumab[17] | Aurexis | mab | humanized | clumping factor A | Staphylococcus aureus infection |
| Telimomab aritox | Fab | mouse | ? | ? | |
| Tenatumomab[6] | mab | mouse | tenascin C | cancer | |
| Teneliximab[13] | mab | chimeric | CD40 | ? | |
| Teplizumab[24] | mab | humanized | CD3 | diabetes mellitus type 1 | |
| Teprotumumab[104] | mab | human | CD221 | hematologic tumors | |
| TGN1412 | ? | humanized | CD28 | chronic lymphocytic leukemia, rheumatoid arthritis | |
| Ticilimumab (= tremelimumab) | mab | human | CTLA-4 | cancer | |
| Tildrakizumab[105] | mab | humanized | IL23 | immunologically mediated inflammatory disorders | |
| Tigatuzumab[6] | mab | humanized | TRAIL-R2 | cancer | |
| TNX-650 | ? | humanized | IL-13 | Hodgkin’s lymphoma | |
| Tocilizumab[4] (= atlizumab) | Actemra, RoActemra | mab | humanized | IL-6 receptor | rheumatoid arthritis |
| Toralizumab[13] | mab | humanized | CD154 (CD40L) | rheumatoid arthritis, lupus nephritis etc. | |
| Tositumomab | Bexxar | ? | mouse | CD20 | follicular lymphoma |
| Tovetumab[106] | mab | human | CD140a | cancer | |
| Tralokinumab[107] | mab | human | IL-13 | asthma etc. | |
| Trastuzumab | Herceptin | mab | humanized | HER2/neu | breast cancer |
| TRBS07[108] | Ektomab | 3funct | ? | GD2 | melanoma |
| Tregalizumab[15] | mab | humanized | CD4 | ? | |
| Tremelimumab | mab | human | CTLA-4 | cancer | |
| Tucotuzumab celmoleukin[33][83] | mab | humanized | EpCAM | cancer | |
| Tuvirumab | ? | human | hepatitis B virus | chronic hepatitis B | |
| Ublituximab[15] | mab | chimeric | MS4A1 | cancer | |
| Urelumab[109] | mab | human | 4-1BB | cancer etc. | |
| Urtoxazumab[4] | mab | humanized | Escherichia coli | diarrhoea caused by E. coli | |
| Ustekinumab[5] | Stelara | mab | human | IL-12, IL-23 | multiple sclerosis, psoriasis, psoriatic arthritis |
| Vantictumab[110] | mab | human | Frizzled receptor | cancer | |
| Vapaliximab[13] | mab | chimeric | AOC3 (VAP-1) | ? | |
| Vatelizumab[20] | mab | humanized | ITGA2 | ? | |
| Vedolizumab | mab | humanized | integrin α4β7 | Crohn’s disease, ulcerative colitis | |
| Veltuzumab[6] | mab | humanized | CD20 | non-Hodgkin’s lymphoma | |
| Vepalimomab | mab | mouse | AOC3 (VAP-1) | inflammation | |
| Vesencumab[15] | mab | human | NRP1 | ? | |
| Visilizumab[46] | Nuvion | mab | humanized | CD3 | Crohn’s disease, ulcerative colitis |
| Volociximab[16] | mab | chimeric | integrin α5β1 | solid tumors | |
| Vorsetuzumab mafodotin[111] | mab | humanized | CD70 | cancer | |
| Votumumab | HumaSPECT | mab | human | tumor antigen CTAA16.88 | colorectal tumors |
| Zalutumumab[16] | HuMax-EGFr | mab | human | EGFR | squamous cell carcinoma of the head and neck |
| Zanolimumab[4] | HuMax-CD4 | mab | human | CD4 | rheumatoid arthritis, psoriasis, T-cell lymphoma |
| Zatuximab[36] | mab | chimeric | HER1 | cancer | |
| Ziralimumab[46] | mab | human | CD147 (basigin) | ? | |
| Zolimomab aritox | mab | mouse | CD5 | systemic lupus erythematosus, graft-versus-host disease |