What is Cancer?
Cancer is characterized as the uncontrollable growth of abnormal cells. All normal cells grow, divide and die in a tightly regulated fashion; however, cancer cells are capable of avoiding apoptosis and outliving normal cells. A tumour begins when one cell becomes cancerous after a mutation occurs at the DNA level of a protooncogene or tumour suppressor, allowing for the cell to overcome the cell cycle check points. The cancerous cell then divides uncontrollably and amounts to a tumour. The cancer can develop more mutations as growth occurs, making the tumour even more dangerous and difficult to control. Cancer cells often develop the ability to begin angiogenesis, which is the proliferation of a network of blood vessels into a cancerous tumour, supplying oxygen and nutrients while removing waste products.
There are two types of tumours: benign and malignant. Benign tumours are rarely life threatening and often non-cancerous. They are confined to one area of the body and do not spread. Malignant tumours are cancerous and have the ability to spread to other parts of the body. They degrade the extracellular matrix and spread throughout the body via the lymph nodes. They can then settle to other parts of the body and result into a new tumour, called a secondary tumour or metastasis. For example, if a man with prostate cancer has new tumour that metastized into his liver, it is called metastatic prostate cancer.
What leads to cancer? Well, there are several possibilities:
1. Genetic predisposition: Many types of cancer often run in families. Some examples are colon cancer and breast cancer. However, if many family members have developed cancer, it does not necessarily mean that you will develop it also. It only means that you are at higher risk. Recognizing that you have a genetic predisposition while using preventative medicine can help you manage or even prevent cancer.
2. Estrogen Exposure: A woman is at higher risk for breast or uterine cancer if she has been exposed to too much estrogen. Estrogen stimulates cell proliferation in the breast and uterus. You may have been exposed to too much estrogen if you began menstruation at an early age or menopause at a late age. Women who have had a child before the age of 35 are at lower risk for developing estrogen related cancer.
3. Ionizing radiation, UV radiation, carcinogenic chemicals, tobacco smoke and alcohol: These 5 substances are carcinogenic since they have the ability to cause DNA damage. Ionizing radiation may come from Xrays or nuclear radiation. UV radiation is from too much exposure to the sun. As for carcinogenic chemicals, some common ones are abestos, benzene, or formaldehyde.
4. Carcinogenic foods: Some carcinogenic foods are salted foods, pickled or smoked foods. Other foods that should be eliminated from the diet are charred meats. Taking vitamin C may prevent cancer by protecting the body from the effects of carcinogenic foods.
5. An unhealthy diet: A diet in high saturated fat is associated with many cancers such as colon, rectum and prostate cancer. Free radicals are generated by the body in a number of ways and are damaging to DNA. Antioxidants reduce the damage that free radicals can do to the body. Therefore, for a healthier diet, take your vitamins and eat lots of antioxidant-containing fruits and vegetables.
What is Chemotherapy?
Chemotherapy is the use of chemical substances to treat diseases. Today, it is commonly used to treat cancer. There are more than 200 types of cancers and over 50 types of drugs. In most cases, cancer chemotherapy works by halting the cell cycle of dividing cells. Cancerous cells are known to grow and reproduce very quickly, therefore chemotherapy is very useful to keep cancer cells from dividing and spreading. The downside is that these chemicals aren’t very specific and can also be taken up by normal cells. Once these normal cells want to divide, they can also be killed. Quick dividing cells that are most affected by chemotherapy are the cells found in the lining of the mouth, the bone marrow, hair follicles and digestive system. Therefore, the most common side effects from these drugs are loss of hair, low immune system activity, low red blood cell count, mouth sores and loss of appetite.
The majority of the chemotherapy drugs can be divided into 7 subtypes: alkylating agents, antimetabolites, anthracyclines, plant alkyloids, topoisomerase inhibitors, monoclonal antibodies, and other antitumour agents. These drugs are known to halt cell division or DNA synthesis in some manner; hence, they halt the cell cycle. For more information, see ‘Types of Chemotherapy’ below.
Chemotherapy can be given to either prolong the life of a palliative patient or to try and cure someone of their cancer. A chemotherapy drug may be given on its own or in combination with other drugs. It may also be given in combination with radiation or surgery. See ‘Treatment Schemes’ below for more details.
Most chemotherapy drugs are administered intravenously while fewer drugs are taken orally (e.g. melphalan). There are also cases where drugs are administered through isolated limb perfusion. Isolated limb perfusion is a technique where the drug is delivered directly to its target (the arm or leg). Using a tourniquet, the flow of blood from the limb is stopped. The blood from the limb in then pumped into a machine where the addition of the drug takes place, and the treated blood is later returned to the limb. This allows for the person to receive drugs in high concentration exactly where the tumour is present. It also reduces the undesirable side-effects of chemotherapy.
Here is a tourniquet being used to stop the blood flow to the patient’s leg:

Figure 1: A tourniquet being used on a patient’s leg
How does the Cell Cycle Work?
The cell cycle is a series of events that take place as a cell goes through replication. This can be divided into two main parts– interphase and the mitotic phase. It is during interphase that the cell grows and gains nutrients for cell division and replicates its DNA. As can be seen in the diagram below, this includes G1, S, and G2. The mitotic phase is when the cell undergoes cell division. This is the process by which many cells go through in order to create more cells (ie. a fertilized egg into an organism, hair, skin, blood, and some organs).
A closer look at the different phases:
Interphase is composed of G1, S, G2, and Go:
G1 is the first phase, and it is where cell activity continues at a high rate. Here synthesis of various enzymes are created for the next S phase. Many of these are enzymes needed for replication. The length of G1 varies depending on the different types of cells.
S phase starts when the DNA is being replicated. The chromosomes are duplicated, with two sister chromatids each. Here the amount of DNA has doubled even though the ploidy of the cell remains the same. RNA transcription and protein synthesis is very low during this phase. However, histone production is high during this phase since most is produced during this phase. The length or duration of S phase stays relatively constant throughout all different types of cells.
G2 is the phase which lasts until the cell reaches mitosis. A significant amount of protein synthesis occurs during this stage. Much of this includes the production of microtubules that are needed for the process of mitosis. If you inhibit protein synthesis during this phase, it will prevent the cell from going through mitosis.
Go is not included in the above diagram. This phase can be called the post-mitotic phase where cells are quiet and/ or unable to divide. Many non-proliferate cells in multicellular organisms stay in this state for very long periods of time. This is common among cells that are fully differentiated (ie. cells that are for a specific function like heart muscle). Cellular senescence is a state where the cell isn’t able divide, and this can be due to degradation or damage to the cell. It is sometimes an alternative to apoptosis where the cell undergoes self-destruction. Some cells are in Go indefinitely until something triggers or induces them to start replicating. Other cells divide throughout their life and so do not stay in the Go phase.
Mitosis consists of generally 4 different phases–prophase, metaphase, anaphase, and telophase. It is where the cells undergo cell division to become two separate daughter cells. It consists of nuclear and cytoplasmic division.

Figure 2: The Cell Cycle
Cell cycle regulation:
There are various steps in regulating the cell cycle, and these include various proteins. These regulation events happen in an ordered and fairly sequential process. There are two key classes of regulators–cyclins and cyclin-dependent kinases (CDKs).
What is the role of cyclins and CDKs?
CDK’s (cyclin-dependent kinases) are a group of protein kinases that are used to regulate the cell-cycle as well as being involved in transcription and mRNA processing. These protein kinases phosphorylate serine and threonine on proteins. CDK’s are activated when they interact with a protein called a cyclin to create a cyclin-CDK complex. These complexes, through phosphorylation, can then activate or interact with other substrates needed for continuation of the cell-cycle.
As said previously, cyclins (a family of proteins) bind to CDK’s to create a complex. Throughout the cell-cycle, the concentration of cyclins vary in a cyclical way. At low concentrations the cyclin unbind from the CDK, leading to inhibition of th enzyme.
Types of cell cycle inhibitors:
The cip/kip family and the INK4a/ARF (Inhibitor of Kinase 4/Alternative Reading Frame) are two families of genes that are known as tumour supressors since they repress tumour formation. Both of these genes prevent the cell-cycle from continuing on, leading to reduced tumour growth. The first family, cip/kip, includes the genes p21 (cip1), p27 (kip1), and p57 (kip2). These genes are CDK(cyclin dependent kinases) inhibitors. They work by binding and inactivating the cyclin-CDK complexes, thereby stopping the G1 phase in the cell-cycle. Damage to the DNA results in p53 being released, and this in turn activates p21. Transforming Growth Factor B (TGF B) is a growth inhibitor and activates gene p27. Two examples of the gene family INK4a/ARF are p161INK4a and p14arf. The first binds to CDK4, stopping the G1 phase. The second stops p53 degradation, which is a tumour supressor.
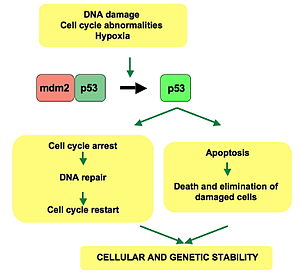
As stated above, p53 is a very well known transcription factor that helps regulate the cell cycle. It is important to many of the cell’s anti-cancer mechanisms. When DNA has sustained damage, p53 activates various DNA repair proteins. Cell senescence is another possibility if there is cell damage; the cell-cycle is held at the G1/S checkpoint. If DNA is too damaged, then p53 can initiate cell apoptosis. p53 is normally inactive in normal and healthy cells. However, it has already been said that if there is DNA damage, this protein is activated and can activate expression of other genes.
Figure 3: p53 mechanisms
Checkpoints in the cell cycle:
Checkpoints are used to monitor and regulate cell cycle progress. These checkpoints make sure that the cell has gone through the necessary phase processes, that everything is working smoothly, and that the DNA is not damaged before allowing the cell-cylce to continue. The G1/S and G2/M checkpoints are the two main checkpoints. G1/S is known as the restriction point. This is the checkpoint right before the cell enters the S phase. The cell either decides to continue dividing, delay dividing, or go into the Go state. The protein p53, which was explained above, plays a major role in many of the controls of checkpoints.
The cell cycle in relation to tumour and cancer growth:
Problems in the cell cycle can lead to uncontrollable regulation and unchecked cell growth. Mutations in the genes of cell cycle inhibitors such as p53 and RB can lead to this uncontrollable cell growth. The cell will continue to go through cell division and mitosis even if the DNA, for example, is severely damaged or mutated. This can lead to further damage and mutations in the cell. When enough mutations arise, a benign tumour may become cancerous. Compared to normal cells, tumour cells are not stopped by the checkpoints–they continue to actively divide. Cells that are going through cell division have exposed DNA that can be damaged by drugs and radiation, leading to cell death. Chemotherapy or radiation can then kill cells that are in this active state. For example, in a process known as debulking (removing just part of the tumour), a large portion of the tumour mass is removed, which forces the other tumour cells to go from the Go stage to G1.
Types of Chemotherapy
There are many different types of drugs used to treat cancer. Each type works in different ways to affect cancerous cells.
Topoisomerase Inhibitors:
These are a type of drug used to inhibit or interfere with topoisomerase enzymes I and/or II. Topoisomerase I works by cutting one strand of DNA, passing the other through it, then reannealing the cut strand. Topoisomerase II, on the other hand, cuts both strands, and passes an unbroken double strand through it then reanneals the cut strand. By inhibiting DNA transcription and replication, these drugs then prevent cancerous cells from replicating.
Types of Topoisomerase I inhibitors: Irinotecan and Topotecan
Types of Topoisomerase II inhibitors: Amsacrine, Etoposide, and Teniposide
Alkylating Agents:
These drugs are not specific to any one area of the cell-cycle and work best when the cell is in the resting (Go) phase. For example, as the name states, Busulfan alkylates the DNA to produce DNA-DNA and DNA-protein cross linking. This then prevents the cell from replicating DNA and syntesizing RNA or proteins. There are many different types of alkylating agents:
Alkylsulfonates: Busulfan
Ethylenimines: Thiotepa and Hexamethylamine
Hydrazines and Triazines: Altretamine, Procarbazine, Dacarbazine, Temozolomide
Metal salts: Carboplatin, Cisplatin, and Oxaliplatin
Mustard gas derivatives: Mechlorethamine, Cyclophosphamide, Chlorambucil, Melphalan, and Ifosfamide
Nitrosureas: Carmustine, Lomustine, and Streptozocin
An example of the mechanism by which these drugs work is shown in the Busulfan mechanism below:

Antitumour Antibiotics (Antineoplastics):
These drugs come from different strains of a soil fungus called Streptomyces, which produces natural products. They work at various cell-cycle phases and are considered cell-cycle specific. Chromomycins interrupt RNA synthesis. For example, Actinomycin-D binds to the DNA transcription initiation complex and stops RNA polymerase. Mitomycin and Bleomycin are examples that work by forming and releasing free radicals into the cell, causing damage.
Anthracyclines: explained below
Chromomycins: Actinomycin-D and Plicamycin
Other: Mitomycin and Bleomycin
Anthracyclines:

Figure 4: Structure of Daunorubicin
These compounds are based upon the compounds daunosamine and naphthacene. Examples of cancers that anthracyclines are used for are leukemias, lymphomas, breast, and lung cancers. These compounds work by inserting themselves between base pairs of DNA and RNA, thereby inhibiting the synthesis of DNA and RNA. By blocking sythesis, tumour cells are prevented from replicating further. They can also cause free oxgen radicals to form, damaging the cell in the process.
Examples include:
Daunorbicin (plus liposomal version)
Doxorubicin (plus liposomal version)
Mitotic Inhibitors/Plant Alkaloids:
These drugs are from various types of plants. They are cell-cycle specific, interrupting cells at different stages of division. An alkaloid is a molecule that is derived from amino acids and is therefore a type of amine. Taxanes can also have biological effects and were originally from the Pacific yew tree. They are di-terpenes (made of four isoprene units with the formula C20H32 ) and work by disrupting the function of microtubules in the cell. There are four general groups of alkaloid drugs.
Vinca alkaloids: Vincristine, Vinblastine, and Vinorelbine
Taxanes: Paclitaxel and Docetaxal
Podophyllaotoxins: Etoposide and Tenisopide
Camnpotothecan analogs: Irinotecan and Topotecan
Figure 5: Etoposide
Monoclonal Antibodies:

Figure 6: The use of monoclonal antibodies to treat tumour cells
Another type of drug treatment includes monoclonal antibodies (mAb). These work by binding to cancer cell-specific antigens, creating an immune response. These antibodies can be modified to deliver toxins, radioisotopes, cytokines, or other compounds to the tumour cell (see figure above). As well, bispecific antibodies can also be made. These can bind their Fab (fragment antigen binding) region to a target antigen and to an effector cell (type of lymphocyte that releases antibodies). An intact antibody is capable of binding to cell receptors or proteins with the Fc (fragment, crystallizable) region. Monoclonal antibodies can directly produce affects that cause apoptosis or cell death.

Figure 7: Structure of an Antibody
Antimetabolites:
These are drugs that are very similar to another biological compound (ie. metabolites) but different enough to interrupt and ruin normal cell processes. They are useful in cancer since they disrupt DNA replication, interfering with cell division to cause tumour cell death. Antimetabolites can act as either purines or pyrimidines in the cell. This process is expanded further in the following section: 5-Fluorouracil is an antimetabolite
Other:
A newer agent that would fall into the antitumour agents subgroup is called Gleevec, which is a tyrosine kinase inhibitor. Certain types of cancer such as chronic myelogenous leukemia and gastrointestinal stromal tumors are known to express an abnormally high concentration of tyrosine kinase inhibitors. Tyrosine kinases are proteins that tell the cell to grow and multiply. This drug is therefore specific to these types of cancer, however it may also affect normal cells and give undesirable side-effects such as fluid retention in the lungs, heart failure, liver problems, abnormal bleeding and skin blistering. This drug is administered orally. Other types of drugs that are not in a single category include:
Ribonucleotide reductase inhibitor: Hydroxyurea
Andrenocortical steroid inhibitor: Mitotane
Enzymes: Asparaginase and Pegaspargase
Antimicrotubule agent: Estramustine
Retinoids: Bexarotene, Isotretinoin, Tretinoin (ATRA)
5-Fluorouracil – An Antimetabolite
5-fluorouracil is a common chemotherapeutic drug that has been used for the last 40 years. It is an antimetabolite drug, meaning that it gets incorporated into DNA and RNA. It was discovered in the 1950s following the observation that rat hepatomas used the pyrimadine uracil more rapidly than normal tissues and was a potential target for antimetabolite chemotherapy. 5-FU is a clear drug that is given by an intravenous route. It can also be found as a cream to treat skin cancer. It is commonly used against solid tumours like in breast, head, pancreatic, stomach, neck and colon cancers. It’s most common use is in colorectal cancer. In fact, 5-FU has remained the main agent of treatment for advanced and early-stage colorectal cancers.
5-fluorouracil is an analogue of uracil with a fluorine atom in the carbon-5 position. It is a pyrimidine analog and found to be a thymidylate synthase inhibitor. Using the uracil transporter, 5-FU can enter the cell quite easily. It is then metabolized to three different metabolites that stop the cell cycle: fluorodeoxyuridine monophosphate (FdUMP), fluorodeoxyuridine triphosphate (FdUTP) and fluorouridine triphosphate (FUTP). These metabolites are either incorporated into RNA, DNA, or inhibit thymidylate synthase, which is needed to make thymidine triphosphate for DNA synthesis. Thymidylate synthase in a enzyme used to generate thymidine monophosphate (dTMP). Thymidine monophosphate is then phosphorylated to create thymidine triphosphate, which is used in DNA synthesis.

Figure 8: Thymidylate Synthase making deoxythymidine monophospate

Figure 9: The chemical structure of 5′-fluorouracil
Figure 10: The mechanisms by which 5-FU damages the cell.
5-FU is first converted to fluorouridine monophosphate (FUMP) by orotate phosphoribosyltranferase (OPRT). It is then phosphorylated to create fluorouridine diphosphate (FUDP), which is phosphorylated once again to create fluoridine triphospate (FUTP). It is essentially equivalent to uridine triphosphate, but has a fluorine at carbon 5. Hence, once incorporated, it leads to RNA damage. When FUTP is incorporated into RNA, tRNA and mRNA cannot be translated. To be specific, pre-mRNA cannot be processed into mature mRNA. Polyadenylation and splicing cannot take place. Therefore, rRNA, tRNA, mRNA and snRNA are never processed and the cell must go through apoptosis. FUDP can also become fluorodeoxyuridine diphosphate by ribonucleotide reductase (RR), which makes a ribonucleotide become a deoxyribonucleotide. Once dephosphorylated to become fluorodeoxyuridine monophosphate (FdUMP), it inhibits thimydylate synthase. Thymidylate synthase is the only enzyme that can can create deoxythimidine monophosphate from deoxyuridine monophosphate. Therefore, if there is a shortage of deoxythimidine monophosphate, no more can be made when TS is inhibited. Hence, DNA synthesis is halted without deoxythimidine triphosphate. It has also been found that UDG (urasil-DNA-glycosylase) is incapable of doing excision and repair during DNA synthesis when the ratio of dUTP to dTTP is too high. Also, if fluorodeoxyuridine diphosphate is phosphorylated into fluorodeoxyuridine triphosphate, it can be incorporated into DNA and damage the cell. All mechanisms listed essentially lead to cell death.
The response rates for 5-FU are low, being around 10-15%. Many patients have a high resistance rate to this drug and overcoming the resistance has been a problem. Its half life is short, being only a few minutes. The reasoning for this is that there is a protein called DPD (dihydropyrimidine dehydrogenase) that converts 5-FU to the DHFU (dihydrofluorouracil). Dihydrofluoricil has no negative effects on the cell and does not induce apoptosis, the wanted effect for cancer chemotherapy. This is the rate limiting step. 80% of the administered drug is metabolized by DPD in the liver, making its half life short. Therefore, high levels of 5-FU are normally administered. People with high level of DPD often become resistant to the drug and combination chemotherapy is needed. However, there are patients with a DPD enzyme deficiency. Since DPD deficient patients are incapable of breaking down the expected amount, severe side effects and sometimes death result. Patients with severe side effects should discontinue their treatment with 5-FU.
Another resistance possibility is high levels of TS expression. Many cell lines that are resistant to 5-FU have been found to have high levels of TS mRNA and protein. The gene for TS is polymorphic and usually has either two TSER2 or three TSER3 28 base pair tandem repeat sequences. Homozygous TSER3 respond less to 5-FU than do homozygous TSER2 or heterozygous TSER2/TSER3 patients. Homozygous TSER3 patients express 3 times more TS mRNA than do homozygous TSER2 patients.
The last discovered resistance problem for 5-FU is the possibility for loss of p53 function. When DNA is damaged, p53 causes the expression of CDKN1A and GADD45alpha, arresting the cell cycle. In severe damage cases, it can also induce the expression of FAS and BAX which result in cell apoptosis. If a patient with colorectal cancer has a tumour without p53 function, the cell may not be told to arrest or die and will continue to proliferate, even if severely damaged. Such cases are insensitive to 5-FU.
To decrease resistance in patients with high levels of TS, leucovorin is usually administered. It turns out that for optimal 5-FU inhibition of thymidylate transferase, high levels of reduced folate CH2TF are necessary. CH2TF helps the metabolite FdUMP bind to TS. Leucovorin enters the cell by the reduced folate carrier and is anabolized to create CH2TF. It is then polyglutamated to enhance the stability. If the molecule is polyglutamated up to 5 times, 5-FU is actually 5 times more potent! The response in patients using leucovorin and 5-FU increased from 11% to 23% response rate.
Another mechanism that works to decrease resistance to 5-FU is inhibiting DPD. Such a drug happens to be Ptorafur which is uracil in combination with 5-FU. It has a 4:1 ratio of uracil to 5-FU. The idea is to saturate DPD with as much uracil (its natural substrate) as possible to overcome its affinity for 5-FU. 5-FU, in combination with Ptorafur and leucovorin happen to increase response rates from 13% to 94% in rat models. All 3 drugs in combination have yet to be used on humans.
Fluorouracil is an S phase specific drug. As a pyrimidine analog, it is metabolized by the cell to create different cytotoxic metabolites. These metabolites are then incorporated into the DNA. If the cell is incapable of reproducing, the cell cycle arrests and apoptosis is induced.
Some adverse side effects to the drug include myelosuppression (low blood cell count due to dying bone marrow cells), mucositis(inflammations of the mucous membrane in the GI tract), dermatitis (inflammation of the skin), diarrhea, watery eyes, soreness of the mouth and cardiac toxicity.
Treatment Schemes
Chemotherapy drugs can be given using different strategies. They can be used to either cure cancer, prolong life, or to help ease the patient’s symptoms during cancer (palliative). Using drugs with other treatments such as surgery or radiation is called combined modality chemotherapy, and this is the most common form of treatment today. Chemotherapy drugs can be given either before or after surgery or radiation. Radiation therapy is where high doses of radiation are used to kill cancerous cells and prevent them from spreading. Radiation can be given externally or internally. External beam radiation therapy is when a machine aims radiation at the cancer, and it is a local treatment, specific to the desired body part. Internal radiation consists of putting the radiation inside the body. This can be done in a solid form or a liquid form. Radiation can help shrink the size of the cancer so that chemotherapy can work better. Likewise, radiation can be given after chemotherapy to make sure all the cancerous cells have died.

Figure 11: The use of radiation to treat cancer.
Surgery consists of manually removing the tumour from the body. Neoadjuvant chemotherapy–preoperative treatment– is where chemotherapy is used to decrease the size of the tumour before surgery can be performed. This is beneficial since it can make the surgery less harmful and more effective. When there is little evidence of cancer but a possibility of recurrence, adjuvant chemotherapy–postoperative treatment–may be used. Not only can it reduce the chance of resistance by the cells, but it is useful in killing cancerous cells that may have spread to other parts of the body. This can be very effective due to the succeptibility of newly growing tumours, which are dividing rapidly.
Using different drugs together is called combination chemotherapy. The benefit of this method is that cells have a lower chance of becoming resistant to any one drug. Also, different combinations of drugs are used for different types of cancers; each cancer may have a certain combination of drugs that provide the best results for that particular cancer. For example, AC chemotherapy is one combination used to treat breast cancer. The AC stands for the names of the drugs: doxorubicin (originally known as Adriamycin®) and cyclophosphamide. Another example is BEP, used for testicular cancer. The drugs used are bleomycin, etoposide, and cisplatin.
Palliative chemotherapy is when a cure is not expected. Instead, it is used to decrease tumour size and increase the length of time that a patient may live. It may also be used to ease the symptoms caused by the tumour and improve the quality of life. In this case, even though the drug may cause side-effects, it is outweighed by relief from tumour symptoms.
Side Effects
As mentioned earlier, cancer chemotherapy is often nonspecific and can kill fast-dividing normal cells. The cells that are most commonly affected by chemotherapy are the hair follicle cells, the mucosal cells from the GI tract, the cells lining the inside of the mouth, cells producing nail and skin and cells in the bone marrow. Since every patient is different, some patients may experience mild side effects while others experience severe. Side effects may also worsen as treatment progresses or vice versa. Every drug has its own specific side effects but most patients do not experience every side effect listed. Every patient is different and everyone reacts to different drugs in their own specific manner. When treatment ceases, the normal cells begin to regenerate themselves and side effects usually cease. As for the cancer cells however, hopefully they were killed during chemotherapy and do not regenerate.
Most common side effects found in the mouth are mouth sores or ulcers. They can become apparent as soon as 5 to 10 days after beginning therapy. Pain killers and antibiotics should be administered to decrease the pain symptoms in the mouth and to prevent infection within the ulcers. Sometimes, taste can also be affected. Certain foods may taste more ‘metallic’. However, taste should return to normal after treatment has ceased. Acidic foods should be avoided to keep the mouth ulcers from becoming worse.

Figure 12: Mouth ulcers and sores are common with chemotherapy.
The most common side effects with the GI tract are loss of appetite, diarrhea, constipation or ulcers. Chemotherapy can kill the mucosal cells or irritate them in your GI tract. Therefore, absorption of water may be difficult in your small intestine, and diarrhea often results. If the patient is experiencing extreme diarrhea, the doctor should be warned immediately so that an IV may be administered. Patients who experience constipation usually have affected nerve supply. The nerves don’t allow the patient to know he needs to use the washroom and constipation results. However, this side effect doesn’t usually last very long and it is very uncommon. Drinking large amounts of fluids also helps to reverse the problem.

Figure 13: Stomach ulcers are a common side effect with chemotherapy.
As for skin, sensitive skin and blisters are the most common side effect. Nails can often become brittle as well. Cream, non-chlorinated water, soft soaps and sun block can make the side effects more bearable.

Figure 14: Here is a picture of how nails often degenerate with chemotherapy.
Hair loss is the most well known side effect. Hair thinning, hair loss in patches, complete hair loss or brittle hair are common results of chemotherapy. Using a cold cap sometimes reduces hair loss, depending on the drug of use. A cold cap is worn on the patients head which reduces blood flow to the scalp, hence less drug circulated to the scalp and therefore, fewer hair follicles are affected. Some doctors are against this use however because if cancer cells are present in the scalp, they will not be affected by the chemotherapy.

Figure 15: Hair loss is a common side effect with chemotherapy.
Bone marrow contains the stem cells used to create red blood cells, white blood cells and platelets. The stem cells are fast dividing, and are commonly affected by chemotherapy. Low red blood cell counts lead to the patient’s muscles and body parts having a low amount of oxygen. The patient will often have a low energy level and can also become anaemic. Blood transfusions are often used to treat the anaemia. Drugs containing erythropoeitin, which stimulate red blood cell production, can also be administered. Neutropenia is a condition characterized by a low white blood cell level. This can be dangerous since white blood cells are needed to fight off infection. Antibiotics can also be administered to decrease the patient’s chances of being sick. With low platelet count, bruising and nosebleeds become more common. Platelet transfusions are administered to make up for the loss.
References
“5-Fluorouracil: mechanisms of action and clinical strategies” Journal of Nature Reviews. Cancer. May 2003, Volume 3, pages 330-338
http://en.wikipedia.org/wiki/chemotherapy
http://en.wikipedia.org/wiki/Cell_cycle
http://www.cancerbackup.org.uk/Treatments/Chemotherapy/Generalinformation/Howitworks
http://www.cancer.gov/Templates/db_alpha.aspx?CdrID=44853
http://www.aclstudygroup.com/Images/acl_tourniquet.JPG
http://en.wikipedia.org/wiki/fluorouracil
http://en.wikipedia.org/wiki/Thymidylate_synthase
http://seqcore.brcf.med.umich.edu/mcb500/nucsyl/nucmetab_files/image054.png
http://www.medsafe.govt.nz/Profs/Datasheet/f/FluorouracilEbeweinjpic1.gif
http://www.nature.com/nrc/journal/v3/n5/abs/nrc1074.html;jsessionid=CD7C364304D70640CA73E0ABA8EF5C62
http://en.wikipedia.org/wiki/Monoclonal_antibody
http://www.meds.com/immunotherapy/monoclonal_antibodies.html
http://en.wikipedia.org/wiki/Topoisomerase
http://www.chemocare.com/whatis/types_of_chemotherapy.asp
http://antiagingchoices.com/images/aromatherapy%20images/worsening_nail.jpg
http://en.wikipedia.org/wiki/Anthracycline
http://www.drugguide.com/classification_articles/antineoplastics.htm
http://en.wikipedia.org/wiki/Taxane
http://www.cancer.ca/vgn/images/portal/cit_86751114/36/15/1816216925cw_2007stats_en.pdf
http://www.cancerhelp.org.uk/help/default.asp?page=176
http://upload.wikimedia.org/wikipedia/en/thumb/c/c2/Mouth_sore.JPG/290px-Mouth_sore.JPG
http://www.trevorsorbie.com/template_images/fe4ce1ae47725fd0f25509c5946ca6b7_1%20-%20Before..jpg
http://www.medicalook.com/diseases_images/stomach_ulcer2.jpg
http://users.rcn.com/jkimball.ma.ultranet/BiologyPages/C/CellCycle.html
http://www.people.vcu.edu/~asneden/alkaloids.htm
http://www.cellcycles.org/showabstract.php?pmid=9925645
http://en.wikipedia.org/wiki/Cyclin-dependent_kinase
http://en.wikipedia.org/wiki/P53
http://www.esf.edu/efb/course/EFB325/lectures/cellcycl.htm
http://pim.medicine.dal.ca/abfc.htm
http://www.cancer.gov/cancertopics/radiation-therapy-and-you/page2
http://www.cancerbackup.org.uk