Green Chem., 2013, Advance Article
DOI: 10.1039/C3GC41469H, Paper
DOI: 10.1039/C3GC41469H, Paper
Guodong Ding, Weitao Wang, Tao Jiang, Buxing Han
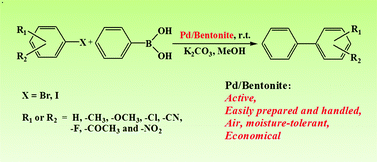
The Pd/bentonite catalyst prepared by a simple impregnation method in water is very active and stable for the Suzuki-Miyaura reaction.
The Pd/bentonite catalyst prepared by a simple impregnation method in water is very active and stable for the Suzuki-Miyaura reaction.
Clays, which are nontoxic, abundant, and cheap, are very promising supports for the design and preparation of green catalysts. In this work, the Pd/bentonite catalyst was fabricated by a simple impregnation method using water as the medium. The catalyst was characterized by powder X-ray diffraction (XRD), Fourier transform infrared spectroscopy (FT-IR), transmission electron spectroscopy (TEM), X-ray photoelectron (XPS) and inductively coupled plasma atomic emission spectroscopy (ICP-AES) techniques. The performance of Pd/bentonite in the Suzuki–Miyaura reaction was studied. It was found that for aryl bromides and iodides with various electron-donating and electron-withdrawing groups such as –CH3, –OCH3, –Cl, –CN, –F, –COCH3 and –NO2, the coupling reaction of substrates with arylboronic acid proceeded smoothly at low catalyst loading (Pd 0.06 mol%) under ambient temperature. The catalyst could be reused at least 7 times without any decrease in activity.