http://www.nature.com/nchem/journal/v3/n2/full/nchem.949.html
Nature chemistry, v3, pg140-145, 2011
DOI:doi:10.1038/nchem.949
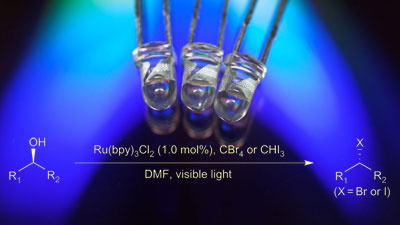
The development of new means of activating molecules and bonds for chemical reactions is a fundamental objective for chemists. In this regard, visible-light photoredox catalysis has emerged as a powerful technique for chemoselective activation of chemical bonds under mild reaction conditions. Here, we report a visible-light-mediated photocatalytic alcohol activation, which we use to convert alcohols to the corresponding bromides and iodides in good yields, with exceptional functional group tolerance. In this fundamentally useful reaction, the design and operation of the process is simple, the reaction is highly efficient, and the formation of stoichiometric waste products is minimized.
A greener way to convert alcohols to their corresponding bromides and iodides using visible light and without generating wasteful by-products has been developed by US researchers. The new method could provide an industrially viable alternative to existing routes.
The transformation of alcohols to their corresponding halides is one of the most widely used reactions in organic synthesis, but traditional methods often require harsh conditions and can generate high levels of undesired stoichiometric waste by-products that are difficult to remove from the reaction mixture.
Corey Stephenson and colleagues at Boston University have converted alcohols to halides using a photocatalyst that absorbs blue light from a light-emitting diode (LED) and polyhalomethanes such as carbon tetrabromide (CBr4) and triiodomethane (CHI3) as the halide source.
The team employed a ruthenium (II) complex to absorb visible light to form an excited ruthenium (II) complex. This ‘excited state’ catalyst acts as a reducing agent by giving up an electron to CBr4 to form a ruthenium (III) complex which the team observed by fluorescence quenching experiments.
The CBr4 then dissociates to form a CBr3![]() radical and Br–. The CBr3
radical and Br–. The CBr3![]() radical then combines with a dimethylformamide (DMF) solvent to produce a solvent active species that goes on to react with the alcohol to form the resulting halide at yields of up to 98 per cent.
radical then combines with a dimethylformamide (DMF) solvent to produce a solvent active species that goes on to react with the alcohol to form the resulting halide at yields of up to 98 per cent.
