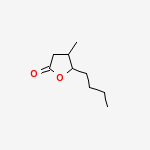
Whisky LactoneWhisky lactone, also known as β-methyl-γ-octalactone or quercus lactone (from the Latin for oak treeQuercus alba), is a flavouring found in American bourbon whiskies, and is also found in all types of oak. The flavour gets into the whisky when it’s matured in oak barrels. The pure molecule has a fierce, strong, and sweet smell and can be dissolved in alcohol in any proportion.
6 http://www.sciencedirect.com/science/article/pii/S0957416698000809 5-butyl-4-methyloxolan-2-one | CAS Registry Number: 39212-23-2
The cis isomer is the chemical extracted from oak wood that gives whiskey a coconut-like aroma. But not all isomers of this molecule are quite this tasty. The trans isomers of 3-methyl-4-octanolide is by contrast, used as an insect repellent.
Physical data of the obtained cis-whiskey lactone are
|

http://www.chemistryviews.org/details/ezine/5392681/Amide_Hydrogenation_in_Flow_Reactor.html
Amide Hydrogenation in Flow Reactor (wiley)
Amines are produced by amide hydrogenation over a bimetallic platinum–rhenium catalyst in a high-throughput vertical flow reactor
]]>
The use of alternative reaction solvents is reviewed in terms of life cycle. Supercritical CO2, ionic liquids, fluorous solvents, water, and renewable organics are compared on the basis of their solvency, ease of use, reusability, health and safety, environmental impact, and economic cost.
James H. Clark * and Stewart J. Tavener
Green Chemistry Centre, Department of Chemistry, University of York, Heslington, York, U.K. YO10 5DD
Org. Process Res. Dev., 2007, 11 (1), pp 149–155
DOI: 10.1021/op060160g
Publication Date (Web): November 4, 2006
http://pubs.acs.org/doi/full/10.1021/op060160g?prevSearch=GREEN%2BSOLVENTS&searchHistoryKey=
This article critically reviewS the use of alternative solvents in chemistry. Rather than follow the well-trodden path of discussing in turn the reactions that have been performed in each major type of alternative solvent, we will instead structure our article in terms of what we consider to be the fundamental issues: life cycle analysis (so as to establish the “green” and sustainability aspects from the outset), solvency (so as to consider what is needed in the application and how the alternatives manage to meet these needs), and application (to consider practical issues in both process and product).
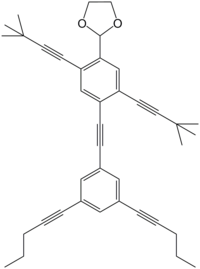
]]>
Systematic name
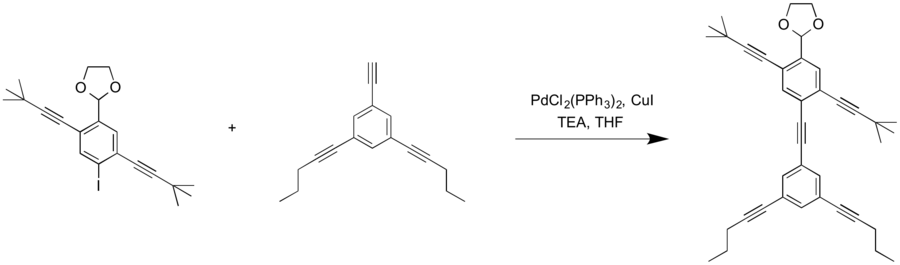
2-(4-{2-[3,5-bis(pent-1-yn-1-yl)phenyl]ethynyl}-2,5-bis(3,3-dimethylbut-1-yn-1-yl)phenyl)-1,3-dioxolane
Other names
1,3-dioxolane, 2-[2,5-bis(3,3-dimethyl-1-butyn-1-yl)-4-[2-(3,5-di-1-pentyn-1-ylphenyl)ethynyl]phenyl]
NanoKid
NanoPutian
618904-86-2 cas


NanoKids Educational Outreach Program]
- “To significantly increase students’ comprehension of chemistry, physics, biology, and materials science at the molecular level.”
- “To provide teachers with conceptual tools to teach nanoscale science and emerging molecular technology.”
- “To demonstrate that art and science can combine to facilitate learning for students with diverse learning styles and interests.”
- “To generate informed interest in nanotechnology that encourages participation in and funding for research in the field.”[3]

Synthesis of NanoKid
Upperbody of NanoKid
Lowerbody of NanoKid
Attachment of Upperbody to Lowerbody of NanoKid
Derivatives of NanoKid
Synthesis of NanoProfessionals[]
Synthesis of the NanoKid in Upright Form]
Synthesis of NanoPutian Chain
References
- ^ a b c d e f g h i Chanteau, S. H.; Tour, J. M. (2003). “Synthesis of Anthropomorphic Molecules: The NanoPutians”. The Journal of Organic Chemistry 68 (23): 8750–8766.doi:10.1021/jo0349227. PMID 14604341. edit
- ^ a b c Chanteau, S. H.; Ruths, T.; Tour, J. M. (2003). “Arts and Sciences Reunite in Nanoput: Communicating Synthesis and the Nanoscale to the Layperson”. Journal of Chemical Education 80 (4): 395. doi:10.1021/ed080p395. edit
- ^ “Welcome to Nanokids.” Accessed May 6, 2013. http://nanokids.rice.edu/.
- ^ “C&EN: EDUCATION – ‘NANOKIDS’ TRY TO GET INTO MIDDLE SCHOOL.” Accessed May 10, 2013. http://pubs.acs.org/cen/education/8214/8214nanokids.html.
- ^ “NanoKids – Mission.” Accessed May 6, 2013. http://cohesion.rice.edu/naturalsciences/nanokids/mission.cfm?doc_id=3039.
External link
http://cohesion.rice.edu/naturalsciences/nanokids/index.cfm
The Tour group designed and synthesized a number of human-shaped organic molecules in this paper. Shown in Figure 1 is a molecule named NanoKid, which was chosen by the group as a basic skeleton. The 3-D model looks like the figure on the right and the structural formula used by chemists is shown on the left. The structural formula might look more human, since the oxygen atoms look kind of like the eyes.


Fig 1 NanoKid
The functional group used for the head part of NanoKid is called acetal. This group is easily exchangeable to make NanoPutians of various occupations (Figure 2). Let’s not be too picky about the bond angles of NanoMonarch and NanoTexan.

Fig 2 various NanoPutians
Unfortunately, NanoBalletDancer seems to be the only one having a different posture (Figure 3). Personally, I would be interested in making NanoPitcher or NanoGermanSuplex!

Fig 3 NanoBalletDancer
The Tour Group also synthesized NanoPutians standing on gold surface with thiol functional groups on their feet, a NanoPutian couple dancing (Figure 4), and even a polymer of NanoPutians (Figure 5).

Fig 4 NanoPutian Couple

Fog 5 NanoPutian polymer
NanoPutians aren’t actually the first example of human-shaped molecule. For example, the molecule shown in Figure 6 has appeared as a joke in a journal published on April Fool’s Day. The molecule shown in Figure 7 has been introduced once as Buddha molecule. Nevertheless, NanoPutians were probably the first case where human-shaped molecules were synthesized systematically(?) to be published as a full paper.


Fig 6 human-shaped molecule Fig 7 molecular Buddha
The Role of NanoPutians
Besides being human-shaped, the NanoPutian molecules have neither notable properties nor potential usefulness for future. The synthesis is also too straightforward to make any significant methodological contribution to chemical science.
Then how did this research get funded and get to be published on Journal of Organic Chemistry? It turns out that the synthesis was a part of the chemistry education program at Rice University aimed at introducing nanotechnology to young students. In fact, it has also been on the cover page of Journal of Chemical Education too. It’s funny though, to imagine the faces of the journal editors when they first read the paper.
But come to think of it, molecules like dodecahedrane and kekulene might not be so different in terms of not having much to appeal other than their structural beauty. Even “total synthesis of biologically active natural products”, the most respected subfield of organic chemistry, has been criticized on its meaning recently. In a way, the NanoPutian research seems to me as a voice saying “synthetic targets should be selected more freely” and almost as an antithesis against the state of organic chemistry today.
Anyway, this paper was introduced by general media and was also one of the topics that received most feedbacks on my homepage. There were those who dismissed it as a meaningless play by chemists, but in terms of directing public interest toward organic chemistry wasn’t it a hundred times more effective than ordinary researches? I think it was an excellent work for the education of young chemists as well.
Professor Tour’s playful sense of molecular design can be seen in his research of NanoCars too, which I will introduce in a separate column. This is a wonderful work which can impress both serious scientists and general public.

nanohippie

nanorobot
– See more at: http://organicchemistrysite.blogspot.in/#sthash.KDZiGxlJ.dpuf
]]>
capecitabine
154361-50-9
- R-340, Ro-09-1978, Xeloda
pentyl [1-(3,4-dihydroxy-5-methyltetrahydrofuran-2-yl)-5-fluoro-2-oxo-1H-pyrimidin-4-yl]carbamate

MONDAY Sept. 16, 2013 — The first generic version of the oral chemotherapy drug Xeloda (capecitabine) has been approved by the U.S. Food and Drug Administration to treat cancers of the colon/rectum or breast, the agency said Monday in a news release.
This year, an estimated 142,820 people will be diagnosed with cancer of the colon/rectum, and 50,830 are predicted to die from the disease, the FDA said, citing the U.S. National Cancer Institute. An estimated 232,340 women will be diagnosed with cancer of the breast this year, and some 39,620 will die from it.
The most common side effects of the drug are diarrhea, vomiting; pain, redness, swelling or sores in the mouth; fever and infection, the FDA said.
The agency stressed that approved generics have the same high quality and strength as their brand-name counterparts.
License to produce the generic drug was given to Israel-based Teva Pharmaceuticals. The brand name drug is produced by the Swiss pharma firm Roche.
Capecitabine (INN) /keɪpˈsaɪtəbiːn/ (Xeloda, Roche) is an orally-administeredchemotherapeutic agent used in the treatment of metastatic breast andcolorectal cancers. Capecitabine is a prodrug, that is enzymatically converted to 5-fluorouracil in the tumor, where it inhibits DNA synthesis and slows growth of tumor tissue. The activation of capecitabine follows a pathway with three enzymatic steps and two intermediary metabolites, 5′-deoxy-5-fluorocytidine (5′-DFCR) and 5′-deoxy-5-fluorouridine (5′-DFUR), to form 5-fluorouracil

Indications
Capecitabine is FDA-approved for:
- Adjuvant in colorectal cancer Stage III Dukes’ C – used as first-line monotherapy.
- Metastatic colorectal cancer – used as first-line monotherapy, if appropriate.
- Metastatic breast cancer – used in combination with docetaxel, after failure of anthracycline-based treatment. Also as monotherapy, if the patient has failed paclitaxel-based treatment, and if anthracycline-based treatment has either failed or cannot be continued for other reasons (i.e., the patient has already received the maximum lifetime dose of an anthracycline).
In the UK, capecitabine is approved by the National Institute for Health and Clinical Excellence (NICE) for colon and colorectal cancer, and locally advanced or metastatic breast cancer.[1] On March 29, 2007, the European Commission approved Capecitabine, in combination with platinum-based therapy (with or without epirubicin), for the first-line treatment of advanced stomach cancer.
Capecitabine is a cancer chemotherapeutic agent that interferes with the growth of cancer cells and slows their distribution in the body. Capecitabine is used to treat breast cancer and colon or rectum cancer that has spread to other parts of the body.
Formulation
Capecitabine (as brand-name Xeloda) is available in light peach 150 mg tablets and peach 500 mg tablets.
- Lacy, Charles F; Armstrong, Lora L; Goldman, Morton P; Lance, Leonard L (2004). Lexi-Comp’s Drug Information Handbook (12th Edition). Lexi-Comp Inc. ISBN 1-59195-083-X
- Fischer, David S; Knobf, M Tish; Durivage, Henry J; Beaulieu, Nancy J (2003). The Cancer Chemotherapy Handbook (6th Edition). Mosby. ISBN 0-323-01890-4
- Thomson Centerwatch: Drugs Approved by the FDA (Xeloda) Retrieved 6/05
- Mercier C, Ciccolini J (2007). “Severe or lethal toxicities upon capecitabine intake: is DPYD genetic polymorphism the ideal culprit?”. Trends in pharmacological sciences 28 (12): 597–598.doi:10.1016/j.tips.2007.09.009. PMID 18001850.
- “Subtopics”. Nice.org.uk. Retrieved 2012-08-15.
- Fingerprints May Vanish With Cancer Drug – US News and World Report
- Cancer Drug Erases Man’s Fingerprints – CNN
- “Stritch School of Medicine”. Stritch.luc.edu. Retrieved 2012-08-15.
- Xeloda.com (patient information, tools, and resources)
- OralChemo Advisor (patient information)
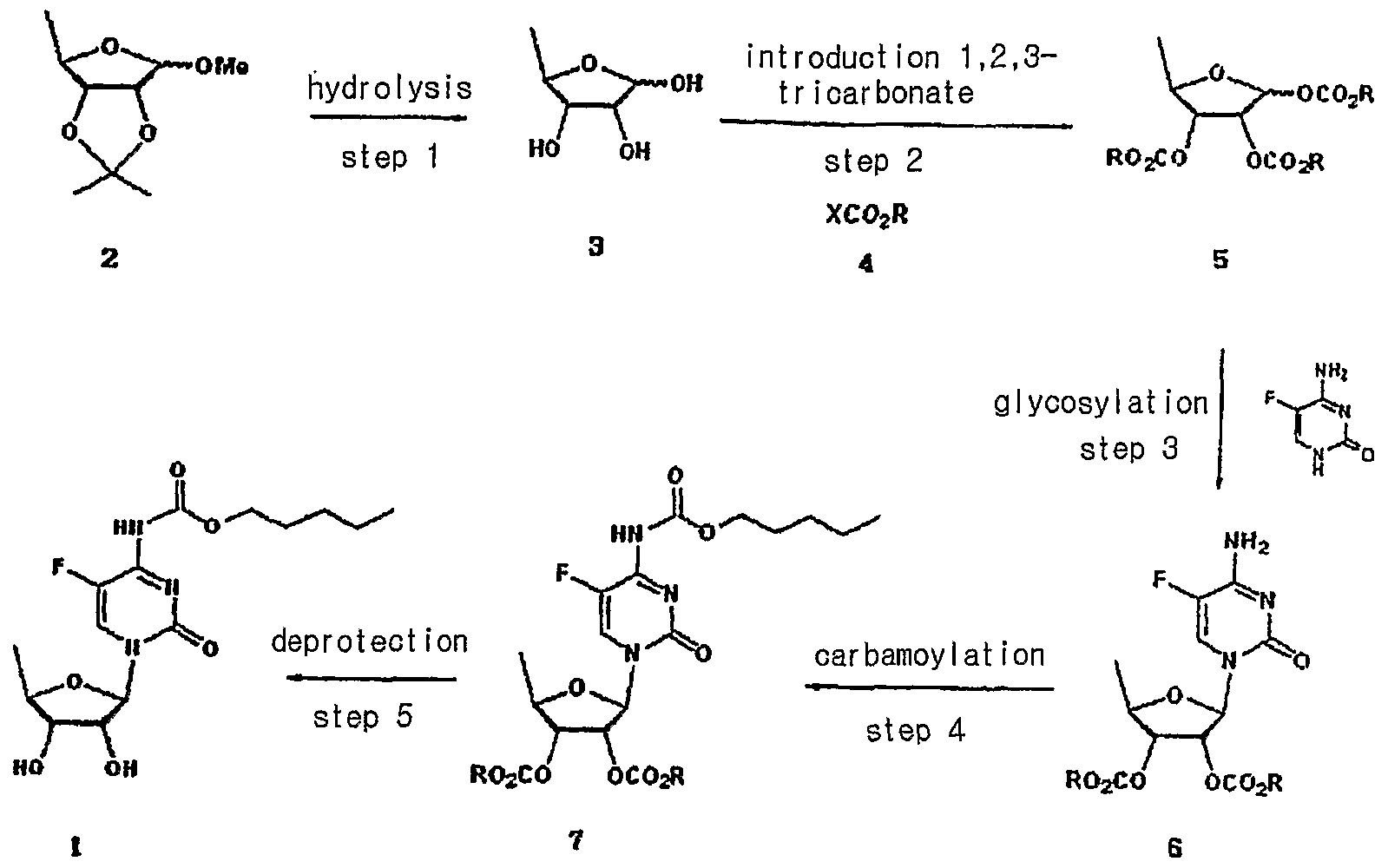
Capecitabine is an orally-administered anticancer agent widely used in the treatment of metastatic breast and colorectal cancers. Capecitabine is a ribofuranose-based nucleoside, and has the sterochemical structure of a ribofuranose having an β-oriented 5-fluorocytosine moiety at C-I position.
US Patent Nos. 5,472,949 and 5,453,497 disclose a method for preparing capecitabine by glycosylating tri-O-acetyl-5-deoxy-β-D-ribofuranose of formula I using 5-fluorocytosine to obtain cytidine of formula II; and carbamoylating and hydrolyzing the resulting compound, as shown in Reaction Scheme 1 :
Reaction Scheme 1
1
The compound of formula I employed as an intermediate in Reaction
Scheme 1 is the isomer having a β-oriented acetyl group at the 1 -position, for the reason that 5-fluorocytosine is more reactive toward the β-isomer than the α-isomer in the glycosylation reaction due to the occurrence of a significant neighboring group participation effect which takes place when the protecting group of the 2-hydroxy group is acyl.
Accordingly, β-oriented tri-O-acetyl-5-deoxy-β-D-ribofuranose (formula
I) has been regarded in the conventional art to the essential intermediate for the preparation of capecitabine. However, such a reaction gives a mixture of β- and α-isomers from which cytidine (formula II) must be isolated by an uneconomical step.
Meanwhile, US Patent No. 4,340,729 teaches a method for obtaining capecitabine by the procedure shown in Reaction Scheme 2, which comprises hydrolyzing 1-methyl-acetonide of formula III to obtain a triol of formula IV; acetylating the compound of formula IV using anhydrous acetic anhydride in pyridine to obtain a β-/α-anomeric mixture of tri-O-acetyl-5-deoxy-D-ribofuranose of formula V; conducting vacuum distillation to purify the β-/α-anomeric mixture; and isolating the β-anomer of formula I therefrom:
Reaction Scheme 2
III IV
However, the above method is also hampered by the requirement to perform an uneconomical and complicated recrystallization steps for isolating the β-anomer from the mixture of β-/α-anomers of formula V, which leads to a low yield of only about 35% to 40% (Guangyi Wang et al., J. Med. Chem., 2000, vol. 43, 2566-2574; Pothukuchi Sairam et al., Carbohydrate Research, 2003, vol. 338, 303-306; Xiangshu Fei et al., Nuclear Medicine and Biology, 2004, vol. 31, 1033-1041; and Henry M. Kissman et al., J. Am. Chem. Soc, 1957, vol. 79, 5534-5540).
Further, US Patent No. 5,476,932 discloses a method for preparing capecitabine by subjecting 5′-deoxy-5-fluorocytidine of formula VI to a reaction with pentylchloroformate to obtain the compound of formula VII having the amino group and the 2-,3-hydroxy groups protected with C5Hi1CO2 groups; and removing the hydroxy-protecting groups from the resulting compound, as shown in Reaction Scheme 3 :
Reaction Scheme 3
Vl VII 1
However, this method suffers from a high manufacturing cost and also requires several complicated steps for preparing the 5′-deoxy-5-fluorocytidine of formula VI: protecting the 2-,3-hydroxy groups; conducting a reaction thereof with 5-fluorocytosine; and deprotecting the 2-,3-hydroxy groups.
Accordingly, the present inventors have endeavored to develop an efficient method for preparing capecitabine, and have unexpectedly found an efficient, novel method for preparing highly pure capecitabine using a trialkyl carbonate intermediate, which does not require the uneconomical β-anomer isolation steps.
synthesis

more info and description
Aspects of the present invention relate to capecitabine and processes for the preparation thereof.
The drug compound having the adopted name “capecitabine” has a chemical name 5′-deoxy-5-fluoro-N-[(pentyloxy) carbonyl] cytidine and has structural formula I.
H
OH OH I
This compound is a fluoropyrimidine carbamate with antineoplastic activity. The commercial product XELODA tablets from Roche Pharmaceuticals contains either 150 or 500 mg of capecitabine as the active ingredient.
tablets from Roche Pharmaceuticals contains either 150 or 500 mg of capecitabine as the active ingredient.
U.S. Patent No. 4,966,891 describes capecitabine generically and a process for the preparation thereof. It also describes pharmaceutical compositions, and methods of treating of sarcoma and fibrosarcoma. This patent also discloses the use of ethyl acetate for recrystallization of capecitabine. The overall process is summarized in Scheme I.
Scheme I
U.S. Patent No. 5,453,497 discloses a process for producing capecitabine that comprises: coupling of th-O-acetyl-5-deoxy-β-D-hbofuranose with 5- fluorocytosine to obtain 2′,3′-di-O-acetyl-5′-deoxy-5-fluorocytidine; acylating a 2′, 3′- di-O-acetyl-5′-deoxy-5-fluorocytidine with n-pentyl chloroformate to form 5′-deoxy- 2′,3′-di-O-alkylcarbonyl-5-fluoro-N-alkyloxycarbonyl cytidine, and deacylating the 2′ and 3′ positions of the carbohydrate moiety to form capecitabine. The overall process is summarized in Scheme II.
Capecitabine
Scheme Il
The preparation of capecitabine is also disclosed by N. Shimma et al., “The Design and Synthesis of a New Tumor-Selective Fluoropyrimidine Carbamate, Capecitabine,” Bioorganic & Medicinal Chemistry, Vol. 8, pp. 1697-1706 (2000). U.S. Patent No. 7,365,188 discloses a process for the production of capecitabine, comprising reacting 5-fluorocytosine with a first silylating agent in the presence of an acid catalyst under conditions sufficient to produce a first silylated compound; reacting the first silylated compound with 2,3-diprotected-5- deoxy-furanoside to produce a coupled product; reacting the coupled product with a second silylating agent to produce a second silylated product; acylating the second silylated product to produce an acylated product; and selectively removing the silyl moiety and hydroxyl protecting groups to produce capecitabine. The overall process is summarized in Scheme III. te
R: hydrocarbyl
Scheme III
Further, this patent discloses crystallization of capecitabine, using a solvent mixture of ethyl acetate and n-heptane. International Application Publication No. WO 2005/080351 A1 describes a process for the preparation of capecitabine that involves the refluxing N4– pentyloxycarbonyl-5-fluorocytosine with trimethylsiloxane, hexamethyl disilazanyl, or sodium iodide with trimethyl chlorosilane in anhydrous acetonitrile, dichloromethane, or toluene, and 5-deoxy-1 ,2,3-tri-O-acetyl-D-ribofuranose, followed by hydrolysis using ammonia/methanol to give capecitabine. The overall process is summarized in Scheme IV.
Scheme IV
International Application Publication No. WO 2007/009303 A1 discloses a method of synthesis for capecitabine, comprising reacting 5′-deoxy-5- fluorocytidine using double (trichloromethyl) carbonate in an inert organic solvent and organic alkali to introduce a protective lactone ring to the hydroxyl of the saccharide moiety; reacting the obtained compound with chloroformate in organic alkali; followed by selective hydrolysis of the sugar component hydrolytic group using an inorganic base to give capecitabine. The overall process is summarized in Scheme V.
Scheme V
Even though all the above documents collectively disclose various processes for the preparation of capecitabine, removal of process-related impurities in the final product has not been adequately addressed. Impurities in any active pharmaceutical ingredient (API) are undesirable, and, in extreme cases, might even be harmful to a patient. Furthermore, the existence of undesired as well as unknown impurities reduces the bioavailability of the API in pharmaceutical products and often decreases the stability and shelf life of a pharmaceutical dosage form.
nmr
1H NMR(CD3OD) δ 0.91(3H5 t), 1.36~1.40(4H, m), 1.41(3H, d), 1.68~1.73(2H, m), 3.72(1H, dd), 4.08(1H, dd), 4.13~4.21(3H, m), 5.7O(1H, s), 7.96(1H, d)

- The acetylation of 5′-deoxy-5-fluorocytidine (I) with acetic anhydride in dry pyridine gives 2′,3′-di-O-acetyl-5′-deoxy-5-fluorocytidine (II), which is condensed with pentyl chloroformate (III) by means of pyridine in dichromethane yielding 2′,3′-di-O-acetyl-5′-deoxy-5-fluoro-N4-(pentyloxycarbonyl)cytidine (IV). Finally, this compound is deacetylated with NaOH in dichloromethane/water. The diacetylated cytidine (II) can also be obtained by condensation of 5-fluorocytosine (V) with 1,2,3-tri-O-acetyl-5-deoxy-beta-D-ribofuranose (VI) by means of trimethylchlorosilane in acetonitrile or HMDS and SnCl4 in dichloromethane..
-
- EP 602454, JP 94211891, US 5472949.
- Capecitabine. Drugs Fut 1996, 21, 4, 358,
- Bioorg Med Chem Lett2000,8,(7):1697,
- Capecitabine. Drugs Fut 1996, 21, 4, 358,
- EP 602454, JP 94211891, US 5472949.

TiO2 ternary-modified with Fe3+, Ni2+, and Au nanoparticles exhibited a noticeable photocatalytic activity for selective cyclohexane oxidation with O2 under sunlight irradiation.
Ternary modified TiO2 as a simple and efficient photocatalyst for green organic synthesis
E-mail: IDE.Yusuke@nims.go.jp ;
Fax: +81-29-860-4826
DOI: 10.1039/C3CC41174E
Received 13 Feb 2013, Accepted 15 Mar 2013
First published online 15 Mar 2013
]]>
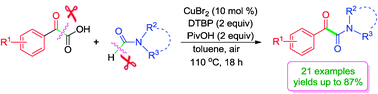
Cu(II)-catalyzed decarboxylative acylation of acyl C–H of formamides with 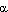 -oxocarboxylic acids leading to
-oxocarboxylic acids leading to 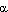 -ketoamides
-ketoamides
| Dengke Li,a Min Wang,*a Jie Liu,a Qiong Zhaoa and Lei Wang*ab |
| Chem. Commun., 2013, 49(35), 3640-3642 |
| http://pubs.rsc.org/en/content/articlelanding/2013/cc/c3cc41188eDOI: 10.1039/C3CC41188E |

E-mail: leiwang@chnu.edu.cn ;
Fax: +86-561-309-0518 ;
Tel: +86-561-380-2069