allow slideshare to load………
]]>

Coenzyme Q10 (ubiquinone-10, CoQ10, CoQ, Q10 or simply Q) is aubiquinone containing 10 isoprenoid units. First discovered in 1957 by Crane et al. [1], its chemical structure was determined by Karl Folkers [2], who later won the Priestley medal from the American Chemical Society. This oil-soluble, vitamin-like micronutrient forms part of the electron transport chain which, in the process of aerobic respiration, generates 95% of the human body’s energy asATP [3].
CoQ, or Q10 is a 1,4-benzoquinone, where Q refers to the quinone chemical group, and 10 refers to the number of isoprenylchemical subunits in its tail.
This oil-soluble, vitamin-like substance is present in most eukaryotic cells, primarily in themitochondria. It is a component of the electron transport chain and participates in aerobic cellular respiration, generating energy in the form of ATP. Ninety-five percent of the human body’s energy is generated this way. Therefore, those organs with the highest energy requirements—such as the heart, liver and kidney—have the highest CoQ10concentrations. There are three redox states of CoQ10: fully oxidized (ubiquinone), semiquinone (ubisemiquinone), and fully reduced (ubiquinol). The capacity of this molecule to exist in a completely oxidized form and a completely reduced form enables it to perform its functions in the electron transport chain and as an antioxidant respectively.
Coenzyme Q10 is synthesized de novo by every cell in the body, but levels decrease with age, in several clinical disorders, and in patients administered certain drugs such as hydroxymethylglutaryl-CoA reductase inhibitors (commonly known as statins). With cardiovascular disease being a leading cause of death in the West, evidence that oral supplements of coenzyme Q10 can benefit patients suffering from heart disease is of increasing appeal. Evidence is also accumulating for its effective treatment of other ailments including mitochondrial disorders and neurodegenerative diseases such as amyotrophic lateral sclerosis (ALS), Huntington’s disease and Parkinson’s disease.
Coenzyme Q10 is one of the best-selling dietary supplements worldwide, available over the counter from health food shops and pharmacies. Its popularity may be due to the wide-ranging claims made for its effectiveness in a myriad of human health issues: it is marketed as an energy booster; a periodontal health promoter; an agent for maintaining normal blood-cholesterol levels; an enhancer of cognitive function; a remedy for hypertension, migraine headaches, radiation injury and cancer; and a superdrug capable of delaying or even reversing the effects of aging. However, perusal of the scientific literature reveals that, while data supporting some claims are forthcoming (such as in the case of heart disease and mitochondrial function), coenzyme Q10 is neither panacea nor elixir [4,5].
References
- Crane, F.L., Hatefi, Y., Lester, R.L. and Widmer, C. (1957) Isolation of a quinone from beef heart mitochondria. Biochim. Biophys. Acta 25, 220–221.
- Wolf, D.E., Hoffman, C.H., Trenner, N.R., Arison, B.H., Shunk, C.H., Linn, B.O., McPherson, J.F. and Folkers, K. (1958) Coenzyme Q. I. Structure studies on the coenzyme Q group. J.Am. Chem. Soc. 80, 4752.
- Ernster, L. and Dallner, G. (1995) Biochemical, physiological and medical aspects of ubiquinone function. Biochim. Biophys.Acta 1271, 195–204.
- Watts, T.L. (1995), Coenzyme Q10 and periodontal treatment: is there any beneficial effect? Br. Dent. J. 178, 209–213.
- European Food Safety Authority Panel on Dietetic Products, Nutrition and Allergies (2010), Scientific Opinion on the substantiation of health claims related to coenzyme Q10 and contribution to normal energy-yielding metabolism (ID 1508, 1512, 1720, 1912, 4668), maintenance of normal blood pressure (ID 1509, 1721, 1911), protection of DNA, proteins and lipids from oxidative damage (ID 1510), contribution to normal cognitive function (ID 1511), maintenance of normal blood cholesterol concentrations (ID 1721) and increase in endurance capacity and/or endurance performance (ID 1913) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J. 8, 1793–1819.

An enone forms an allyl alcohol in a 1,2-addition. Competing conjugate 1,4-addition is suppressed. The solvent is an alcohol such asmethanol or ethanol.
The selectivity can be explained in terms of HSAB theory: carbonyl groups require hard nucleophiles for 1,2-addition. The hardness of the borohydride is increased by replacing hydride groups with alkoxide groups, a reaction catalyzed by the cerium salt by increasing the electrophilicity of the carbonyl group. This is selective for ketones because it is more Lewis basic.
In one application a ketone is selectively reduced in presence of an aldehyde. Actually in presence of methanol as solvent, the aldehyde forms methoxy acetal which is inactive in the reducing conditions.

- References
- Strategic Applications of Named Reactions in Organic Synthesis (Paperback) by Laszlo Kurti, Barbara Czako ISBN 0-12-429785-4
- Lanthanides in organic chemistry. 1. Selective 1,2 reductions of conjugated ketones Jean Louis Luche J. Am. Chem. Soc.; 1978; 100(7); 2226-2227. doi:10.1021/ja00475a040
- Lanthanoids in organic synthesis. 6. Reduction of .alpha.-enones by sodium borohydride in the presence of lanthanoid chlorides: synthetic and mechanistic aspects Andre L. Gemal, Jean Louis Luche J. Am. Chem. Soc.; 1981; 103(18); 5454-5459doi:10.1021/ja00408a029
 Epoxidation
Epoxidation
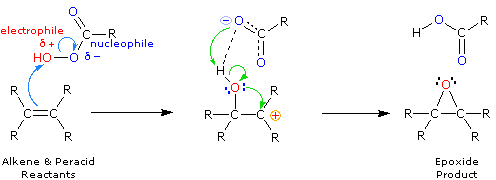 |
 OZONOLYSIS
OZONOLYSIS
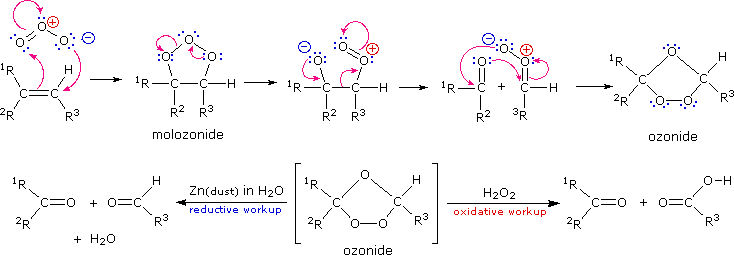
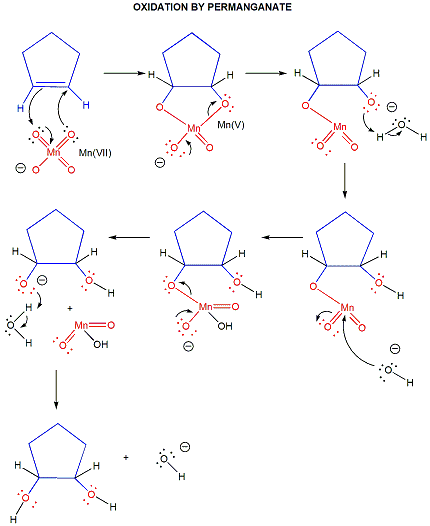
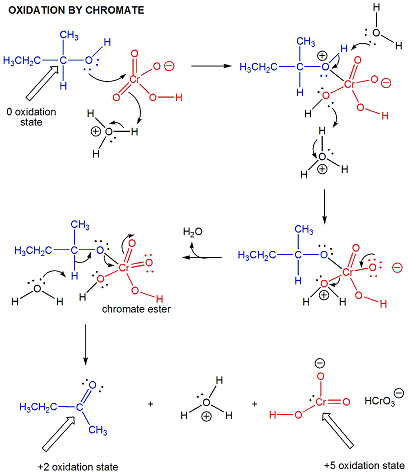
 oxidations
oxidations
 DIELS ALDER RXN
DIELS ALDER RXN
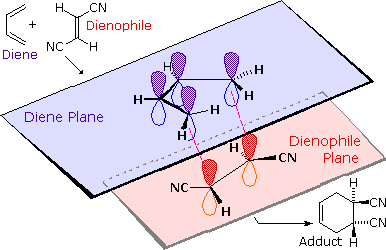
We noted earlier that addition reactions of alkenes often exhibited STEREOSPEFICITY, in that the reagent elements in some cases added syn and in other cases anti to the the plane of the double bond. Both reactants in the Diels-Alder reaction may demonstrate stereoisomerism, and when they do it is found that the relative configurations of the reactants are preserved in the product (the adduct). The following drawing illustrates this fact for the reaction of 1,3-butadiene with (E)-dicyanoethene. The trans relationship of the cyano groups in the dienophile is preserved in the six-membered ring of the adduct. Likewise, if the terminal carbons of the diene bear substituents, their relative configuration will be retained in the adduct. Using the earlier terminology, we could say that bonding to both the diene and the dienophile is syn. An alternative description, however, refers to the planar nature of both reactants and terms the bonding in each case to be suprafacial (i.e. to or from the same face of each plane). This STEREOSPECITY also confirms the synchronous nature of the 1,4-bonding that takes place
 NITRILE HYDROLYSIS UNDER ACIDIC CONDITIONS
NITRILE HYDROLYSIS UNDER ACIDIC CONDITIONS

 NITRILE HYDOLYSIS, BASIC CONDITIONS
NITRILE HYDOLYSIS, BASIC CONDITIONS

