-
Health.com -“There are various explanations why breast-feeding seems to preventbreast cancer,” Gonzalez-Jimenez said. “The most probable of these are …
- read all at
http://news.health.com/2013/08/15/breast-feeding-may-protect-some-women-against-breast-cancer/
By Kathleen Doheny
HealthDay Reporter
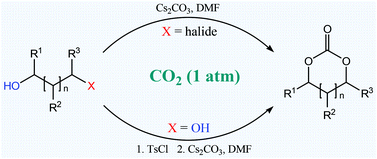
Cyclic carbonates are important compounds for the synthesis of biocompatible polymers and linear dialkyl carbonates, as well as solvents and electrolytes. We report here the synthesis of such compounds from easily accessible starting materials with carbon dioxide as a C1 source and caesium carbonate as the base. This new methodology is able to yield 5- and 6-membered cyclic carbonates very efficiently in a green manner
Synthesis of cyclic carbonates with carbon dioxide and cesium carbonate
E-mail: ygzhang@ibn.a-star.edu.sg ;
Fax: (+65)-6478-9080 ;
Tel: (+65)-6824-7162
Received 26 Apr 2013, Accepted 02 Jun 2013
First published online 04 Jun 2013
]]>

This model shows the role of vesicles, vesicle chains and membrane tubes in M. xanthus biofilms. The scientists believe these connections help cells exchange signals and material. Image: Auer laboratory
Using several imaging techniques, Lawrence Berkeley National Laboratory scientists found that a common soil bacterium stays connected by a network of chain-like membranes. They believe the bacterium uses its network to coordinate social activities-such as evading bacterial enemies and snaring prey-without revealing its location. Even bacteria use social networks
read at
]]> Cyclotides are head-to-tail cyclic peptides in plants
Cyclotides are head-to-tail cyclic peptides in plants
Cyclotides are plant-derived peptides of approximately 30 amino acids. They have the characteristic structural features of a head-to-tail cyclized backbone and a cystine knot arrangement of their three conserved disulfide bonds. Their unique structural features lead to exceptional stability. This and their amenability to chemical synthesis have made it possible to use cyclotides as templates in protein engineering and drug design applications.
David J Craik, University of Queensland, Brisbane, Australia, whose laboratory is working over 20 years in the field, summarizes the history of cyclotides
http://www.chemistryviews.org/details/news/5012211/History_of_Cyclotides.html
more info on cyclotides

Figure 1. Structure and sequence of the prototypic cyclotide kalata B1
Cyclotides are small disulfide-rich proteins that have the unusual feature of a cyclic backbone (hence the name cyclo – peptides). They contain six conserved cystine residues that are arranged in a cystine knot topology in which two disulfide bonds and their connecting backbone segments form an embedded ring in the structure that is penetrated by a third disulfide bond, as shown below.
Cyclotides have a range of interesting biological activities including anti-HIV and neurotensin inhibition, anti-microbial activity and insecticidal activity. They are found in a variety of tropical plants from the Rubiaceae and Violaceae families.
 |
| The structure of kalata B1 showing the distorted beta-sheet topology and the loop nomenclature enabled by the cyclic backbone. |
Cyclotides are small disulfide rich peptides isolated from plants.Typically containing 28-37 amino acids, they are characterized by their head-to-tail cyclised peptide backbone and the interlocking arrangement of their three disulfide bonds. These combined features have been termed the cyclic cystine knot (CCK) motif (Figure 1). To date, over 100 cyclotides have been isolated and characterized from species of the Rubiaceae, Violaceae, and Cucurbitaceae families. Cyclotides have also been identified in agriculturally important families such as the Fabaceae and Poaceae.,
Cyclotides have been reported to have a wide range of biological activities, including anti-HIV, insecticidal, anti-tumour, antifouling, anti-microbial, hemolytic, neurotensinantagonism, trypsin inhibition, and uterotonic activities.[4][5][6] An ability to induceuterine contractions was what prompted the initial discovery of kalata B1.[7]
The potent insecticidal activity of cyclotides kalata B1 and kalata B2 has prompted the belief that cyclotides act as plant host-defence agents (Figure 2). The observations that dozens or more cyclotides may be present in a single plant and the cyclotide architecture comprises a conserved core onto which a series of hypervariable loops is displayed suggest that, cyclotides may be able to target many pests/pathogens simultaneously.
]]>allow slideshare to load………
]]>

Coenzyme Q10 (ubiquinone-10, CoQ10, CoQ, Q10 or simply Q) is aubiquinone containing 10 isoprenoid units. First discovered in 1957 by Crane et al. [1], its chemical structure was determined by Karl Folkers [2], who later won the Priestley medal from the American Chemical Society. This oil-soluble, vitamin-like micronutrient forms part of the electron transport chain which, in the process of aerobic respiration, generates 95% of the human body’s energy asATP [3].
CoQ, or Q10 is a 1,4-benzoquinone, where Q refers to the quinone chemical group, and 10 refers to the number of isoprenylchemical subunits in its tail.
This oil-soluble, vitamin-like substance is present in most eukaryotic cells, primarily in themitochondria. It is a component of the electron transport chain and participates in aerobic cellular respiration, generating energy in the form of ATP. Ninety-five percent of the human body’s energy is generated this way. Therefore, those organs with the highest energy requirements—such as the heart, liver and kidney—have the highest CoQ10concentrations. There are three redox states of CoQ10: fully oxidized (ubiquinone), semiquinone (ubisemiquinone), and fully reduced (ubiquinol). The capacity of this molecule to exist in a completely oxidized form and a completely reduced form enables it to perform its functions in the electron transport chain and as an antioxidant respectively.
Coenzyme Q10 is synthesized de novo by every cell in the body, but levels decrease with age, in several clinical disorders, and in patients administered certain drugs such as hydroxymethylglutaryl-CoA reductase inhibitors (commonly known as statins). With cardiovascular disease being a leading cause of death in the West, evidence that oral supplements of coenzyme Q10 can benefit patients suffering from heart disease is of increasing appeal. Evidence is also accumulating for its effective treatment of other ailments including mitochondrial disorders and neurodegenerative diseases such as amyotrophic lateral sclerosis (ALS), Huntington’s disease and Parkinson’s disease.
Coenzyme Q10 is one of the best-selling dietary supplements worldwide, available over the counter from health food shops and pharmacies. Its popularity may be due to the wide-ranging claims made for its effectiveness in a myriad of human health issues: it is marketed as an energy booster; a periodontal health promoter; an agent for maintaining normal blood-cholesterol levels; an enhancer of cognitive function; a remedy for hypertension, migraine headaches, radiation injury and cancer; and a superdrug capable of delaying or even reversing the effects of aging. However, perusal of the scientific literature reveals that, while data supporting some claims are forthcoming (such as in the case of heart disease and mitochondrial function), coenzyme Q10 is neither panacea nor elixir [4,5].
References
- Crane, F.L., Hatefi, Y., Lester, R.L. and Widmer, C. (1957) Isolation of a quinone from beef heart mitochondria. Biochim. Biophys. Acta 25, 220–221.
- Wolf, D.E., Hoffman, C.H., Trenner, N.R., Arison, B.H., Shunk, C.H., Linn, B.O., McPherson, J.F. and Folkers, K. (1958) Coenzyme Q. I. Structure studies on the coenzyme Q group. J.Am. Chem. Soc. 80, 4752.
- Ernster, L. and Dallner, G. (1995) Biochemical, physiological and medical aspects of ubiquinone function. Biochim. Biophys.Acta 1271, 195–204.
- Watts, T.L. (1995), Coenzyme Q10 and periodontal treatment: is there any beneficial effect? Br. Dent. J. 178, 209–213.
- European Food Safety Authority Panel on Dietetic Products, Nutrition and Allergies (2010), Scientific Opinion on the substantiation of health claims related to coenzyme Q10 and contribution to normal energy-yielding metabolism (ID 1508, 1512, 1720, 1912, 4668), maintenance of normal blood pressure (ID 1509, 1721, 1911), protection of DNA, proteins and lipids from oxidative damage (ID 1510), contribution to normal cognitive function (ID 1511), maintenance of normal blood cholesterol concentrations (ID 1721) and increase in endurance capacity and/or endurance performance (ID 1913) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J. 8, 1793–1819.

 |
|||
|---|---|---|---|
|
|||
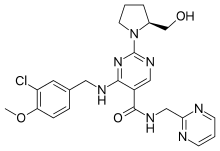
SynthesisAvanafil can be synthesized from a benzylamine derivative and a pyrimidine derivative:[2]
|
|||
 links
links
- Faster-Working Erectile Dysfunction Drug?. CBS News. November 24, 2009.
- Vivus says men taking avanafil were more likely to be ready for sex within 15 minutes. The Gaea Times. January 11, 2010.
- “Avanafil is the New Player in The Erectile Dysfunction Field”. June 28, 2011
- NAME—4-[(3-Chloro-4-methoxybenzyl)amino]-2-[(2S)-2-(hydroxymethyl)-1-p
yrrolidinyl]-N-(2-pyrimidinylmethyl)-5-pyrimidinecarboxamide

 Sanofi announced today that the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) has issued a positive opinion recommending the approval of once-daily Lyxumia® (lixisenatide) for the treatment of adults with type 2 diabetes mellitus to achieve glycemic control in combination with oral glucose-lowering medicinal products and/or basal insulin when these, together with diet and exercise, do not provide adequate glycemic control.
Sanofi announced today that the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) has issued a positive opinion recommending the approval of once-daily Lyxumia® (lixisenatide) for the treatment of adults with type 2 diabetes mellitus to achieve glycemic control in combination with oral glucose-lowering medicinal products and/or basal insulin when these, together with diet and exercise, do not provide adequate glycemic control.
Lixisenatide is a once-daily injectable GLP-1 receptor agonist that has been developed by Sanofi.[1] As of September 2010 it is in clinical trials for diabetes.[2]
Lixisenatide, a glucagon-like peptide-1 agonist (GLP-1), is in development for the treatment of patients with type 2 diabetes mellitus. Lixisenatide was discovered by Zealand Pharma A/S and the global rights are licensed to Sanofi. Lyxumia® is the intended trademark for lixisenatide. Lixisenatide is not currently approved or licensed anywhere in the world.
GLP-1 is a naturally-occurring peptide that is released within minutes of eating a meal. It is known to suppress glucagon secretion from pancreatic alpha cells and stimulate insulin secretion by pancreatic beta cells. GLP-1 receptor agonists are in development as an add-on treatment for type 2 diabetes and their use is endorsed by the European Association for the Study of Diabetes, the American Diabetes Association, the American Association of Clinical Endocrinologists and the American College of Endocrinology.
The GetGoal phase III clinical program will provide data for lixisenatide in adults with type 2 diabetes treated with various oral anti-diabetic agents or insulin. With ten trials in the program, GetGoal started in May 2008 and has enrolled more than 4,300 patients. To date, GetGoal-X, GetGoal-L, GetGoal-L Asia, GetGoal-Mono, GetGoal-S and GetGoal-F1 have reported positive top-line results supporting efficacy and safety for lixisenatide
- Christensen, M; Knop, FK; Holst, JJ; Vilsboll, T (2009). “Lixisenatide, a novel GLP-1 receptor agonist for the treatment of type 2 diabetes mellitus”. IDrugs : the investigational drugs journal 12 (8): 503–13. PMID 19629885.
- http://www.genengnews.com/gen-news-highlights/positive-phase-iii-data-for-two-separate-type-2-diabetes-therapies-reported-at-esad/81243945/
Sanofi Submits Application for Regulatory Approval for Lyxumia
®
(lixisenatide) for the Treatment of Type 2 Diabetes in Japan
 http://en.sanofi.com/Images/30529_20120611_LYXUMIA-JAPAN_en.pdf
http://en.sanofi.com/Images/30529_20120611_LYXUMIA-JAPAN_en.pdf
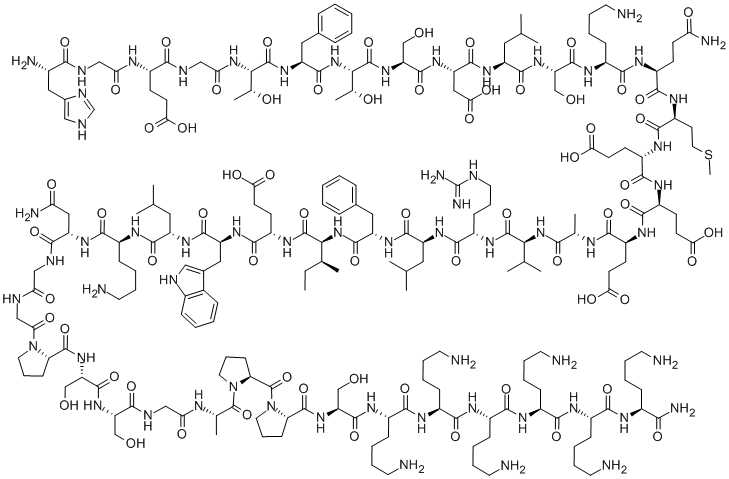
| CAS No. | 320367-13-3 | ||
| Chemical Name: | Lixisenatide | ||
| Synonyms: | lixisenatide | ||
| CBNumber: | CB01518519 | ||
Molecular Formula:
|
C215H347N61O65S |


str from chemblink Online Database of Chemicals from Around the World



 http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/002445/smops/Positive/human_smop_000448.jsp&mid=WC0b01ac058001d127
http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/002445/smops/Positive/human_smop_000448.jsp&mid=WC0b01ac058001d127