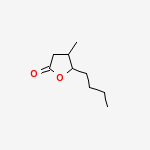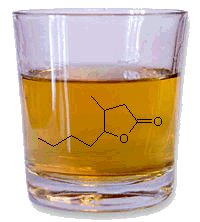
Whisky LactoneWhisky lactone, also known as β-methyl-γ-octalactone or quercus lactone (from the Latin for oak treeQuercus alba), is a flavouring found in American bourbon whiskies, and is also found in all types of oak. The flavour gets into the whisky when it’s matured in oak barrels. The pure molecule has a fierce, strong, and sweet smell and can be dissolved in alcohol in any proportion.
6 http://www.sciencedirect.com/science/article/pii/S0957416698000809 5-butyl-4-methyloxolan-2-one | CAS Registry Number: 39212-23-2
The cis isomer is the chemical extracted from oak wood that gives whiskey a coconut-like aroma. But not all isomers of this molecule are quite this tasty. The trans isomers of 3-methyl-4-octanolide is by contrast, used as an insect repellent.
Physical data of the obtained cis-whiskey lactone are
|
The N-methylation of electron-deficient pyrroles was affected using dimethyl carbonate in the presence of DMF and catalytic DABCO. This alkylation methodology has proven useful for the alkylation of a variety of pyrroles in 72−98% yields and is considered to be greenchemistry relative to the more common use of methyl halides or dimethyl sulfate.

The use of alternative reaction solvents is reviewed in terms of life cycle. Supercritical CO2, ionic liquids, fluorous solvents, water, and renewable organics are compared on the basis of their solvency, ease of use, reusability, health and safety, environmental impact, and economic cost.
James H. Clark * and Stewart J. Tavener
Green Chemistry Centre, Department of Chemistry, University of York, Heslington, York, U.K. YO10 5DD
Org. Process Res. Dev., 2007, 11 (1), pp 149–155
DOI: 10.1021/op060160g
Publication Date (Web): November 4, 2006
http://pubs.acs.org/doi/full/10.1021/op060160g?prevSearch=GREEN%2BSOLVENTS&searchHistoryKey=
This article critically reviewS the use of alternative solvents in chemistry. Rather than follow the well-trodden path of discussing in turn the reactions that have been performed in each major type of alternative solvent, we will instead structure our article in terms of what we consider to be the fundamental issues: life cycle analysis (so as to establish the “green” and sustainability aspects from the outset), solvency (so as to consider what is needed in the application and how the alternatives manage to meet these needs), and application (to consider practical issues in both process and product).
]]>DOI: 10.1039/C3GC41799A, Communication
This synthesis was confirmed to follow the GAP chemistry process, which can avoid traditional chromatography and recrystallization purification methods.
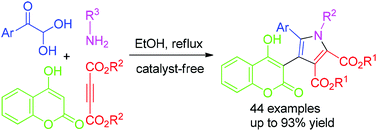
GAP chemistry for pyrrolyl coumarin derivatives: a highly efficient one-pot synthesis under catalyst-free conditions
A concise and efficient one-pot synthesis of pyrrolyl coumarin derivatives via a four-component reaction of 4-hydroxycoumarin, arylglyoxal monohydrate, dialkyl but-2-ynedioate and amines under catalyst-free conditions in an environmentally friendly medium (ethanol) is described. This synthesis was confirmed to follow the group-assisted-purification (GAP) chemistry process, which can avoid traditional chromatography and recrystallization purification methods
spectra
R2 = 3 CHLOROPHENYL
R1= METHYL
dimethyl 1-(3-chlorophenyl)-4-(4-hydroxy-2-oxo-2H-chromen-3-yl)-5-phenyl-1H-pyrrole-2, 3-dicarboxylate (5c).
The reaction of 4-hydroxycoumarin 1 (16.2 mg, 1 mmol), phenylflyoxal monohydrate 2a (15.2 mg, 1 mmol), dimethyl but-2-ynedioate 3a (14.2 mg, 1 mmol) and 3-chloroaniline 4c (12.7 mg, 1 mmol) in ethanol (5 mL), at 80 °C 1.5 h, afforded 46.0 mg (87 %) of 5c.
white powder; m.p.: 242-246°C; IR (KBr, ν, cm-1): 3423, 3075, 3002, 2951, 2853, 1720, 1683,
1577, 1483, 1444, 1303, 1271, 1213, 1126, 1077, 1043, 1013, 988, 924, 880, 761, 698, 649; 1
H NMR (DMSO-d6, 400 MHz): δ 11.29 (s, 1H, OH), 7.76 (d, J = 7.6 Hz, 1H, ArH), 7.55 (t, J = 8.0 Hz, 1H, ArH), 7.40-7.18 (m, 6H, ArH), 7.10-7.02 (m, 5H, ArH), 3.63 (s, 3H, OCH3), 3.60 (s, 3H, OCH3); 13C NMR (DMSO-d6, 75 MHz): δ 164.28, 162.30, 162.20, 161.52, 152.91, 139.00,
138.22, 133.24, 132.88, 130.87, 130.27, 129.99, 129.21, 128.80, 128.44, 128.35, 127.59, 127.38,
124.48, 124.09, 119.89, 116.64, 116.16, 113.35, 98.28, 52.89, 52.18; HRMS (ESI) calcdforC29H2035ClNO7 [M]+: 529.0928, found: 529.0933
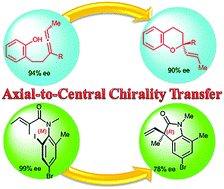
Axial-to-central chirality transfer in cyclization processes
Substrates, bearing axial chirality, can cyclize intra- or inter-molecularly with concomitant transfer of axial-to-central chirality to produce at least one stereocenter. In order to satisfy a strict definition of axial-to-central chirality transfer, the initial axial chirality must be lost during the cyclization process. Highly functionalized enantiopure carbocycles and heterocycles were prepared using this strategy. The transformations of configurationally stable substrates take place with high regio- and stereo-selectivity. Selected examples involving allenes, biaryls, arylamides and transient axially chiral short-lived species are discussed. Special attention is focused on the mechanistic rationale of the chirality transfer.
DOI: 10.1039/C3CS60182J
DOI: 10.1039/C3GC41469H, Paper
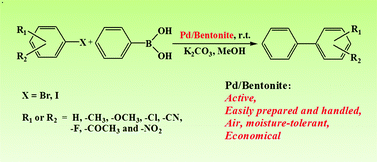
The Pd/bentonite catalyst prepared by a simple impregnation method in water is very active and stable for the Suzuki-Miyaura reaction.
DOI: 10.1039/C3GC40353J, Paper
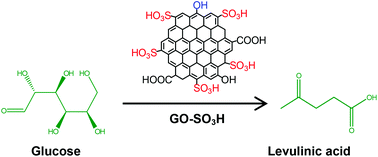
Sulfonated graphene oxide (GO-SO3H) catalyst selectively decomposes glucose to levulinic acid (LA) with excellent yield (around 78%).
Chemical conversion of biomass-derived hexose sugars to levulinic acid over sulfonic acid-functionalized graphene oxide catalysts
Heterogeneous graphene oxide (GO)-based catalysts with sulfonic acid (SO3H) functional groups (GO–SO3H) were used for the selective decomposition of the hexose sugars, glucose and fructose into levulinic acid (LA), which has been used as a platform chemical for various value-added derivatives. The GO–SO3H catalysts gave high yields of around 78% for LA and showed good reuse compatibility with reliable performance. The chemical transformation patterns for hexose sugar decompositions are affected by the temperature, the density of acid sites, and the type of catalyst. The high catalytic performance of GO–SO3H was shown to result from the higher density of Brønsted acid sites in the GO, compared with the Lewis acid sites in other AC–SO3H catalysts. The morphology, surface characteristics, and other physiochemical properties were evaluated using several characterization techniques.

Zeolites modified with aminoalkoxysilanes and cyclodextrin show different uptake and release properties depending on which silane is used
Multifunctional nanocontainers for imaging, targeting, and drug release are a main research area in bio-nanomedicine. Jurriaan Huskens and colleagues, University of Twente, The Netherlands, have functionalized nanoporous zeolite L crystals with β-cyclodextrin (CD) to give multifunctional systems that have the potential for encapsulation of drug molecules inside the zeolite pores and noncovalent attachment of other, for example, targeting, ligand molecules on its surface.
]]>
Image credit: Wikipedia
http://www.forbes.com/sites/daviddisalvo/2012/12/30/top-10-challenges-for-brain-science-in-2013/
]]>
TiO2 ternary-modified with Fe3+, Ni2+, and Au nanoparticles exhibited a noticeable photocatalytic activity for selective cyclohexane oxidation with O2 under sunlight irradiation.
Ternary modified TiO2 as a simple and efficient photocatalyst for green organic synthesis
E-mail: IDE.Yusuke@nims.go.jp ;
Fax: +81-29-860-4826
DOI: 10.1039/C3CC41174E
Received 13 Feb 2013, Accepted 15 Mar 2013
First published online 15 Mar 2013
]]>