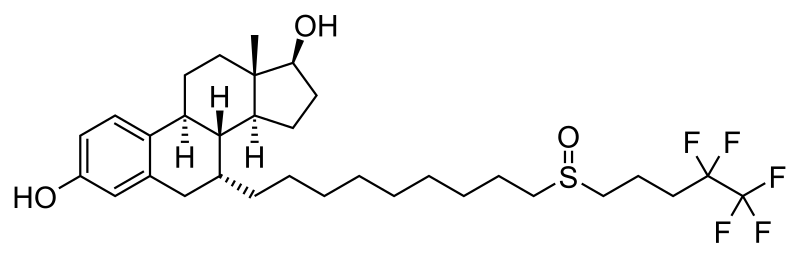
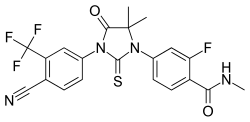
The article is 2012-2013 based and reader discretion is sought to ascertian the stage of approval
Breast Cancer Drugs in Late-Stage Development/Recently Approved

Afinitor® (everolimus)
http://newdrugapprovals.wordpress.com/2013/04/27/drug-spotlight-afinitor-everolimus-novartis/
Sponsor: Novartis
Method of Action: Mammalian target of rapamycin (mTOR) inhibitor
Indications/Phase of Trial: Hepatocellular carcinoma; human epidermal growth factor receptor 2-positive (HER2+) breast cancer first-line and second-line; lymphoma; nonfunctional carcinoid tumor (Phase III; all new indications)
Approved in July in U.S., EU for advanced hormone-receptor-positive (HR+) and human epidermal growth factor Receptor 2-negative (HER2-) metastatic breast cancer with exemestane in postmenopausal women who have already received certain other medicines for their cancer
Approved earlier for adults with pancreatic neuroendocrine tumors (PNET) that cannot be treated with surgery; adults with advanced renal cell carcinoma (RCC) when certain other medicines have not worked; adults with angiomyolipoma, seen with tuberous sclerosis complex (TSC), when surgery is not required immediately; and adults and children with TSC who have a brain tumor called subependymal giant cell astrocytoma (SEGA) that cannot be removed completely by surgery
Avastin (Bevacizumab; RG435)
Sponsor: Roche/Genentech
Method of Action: Monoclonal antibody; Vascular endothelial growth factor (VEGF) inhibitor
Indications/Phase of Trial: U.S.: Relapsed ovarian cancer, platinum-sensitive (Registration); first-line metastatic breast cancer and first-line metastatic ovarian cancer (both Phase III).
EU: Relapsed platinum-resistance ovarian cancer (Phase III)
Metastatic colorectal cancer, treatment beyond progression (Registration); adjuvant breast cancer, HER2- and HER2+; adjuvant NSCLC; first-line glioblastoma (GBM) multiforme; high-risk carcinoid (all Phase III)
Approved for metastatic colorectal cancer (mCRC) when started with the first or second intravenous 5-FU–based chemotherapy for metastatic cancer; advanced nonsquamous non-small-cell lung cancer (NSCLC) with carboplatin and paclitaxel in people who have not received chemotherapy for their advanced disease; metastatic RCC (mRCC) with interferon alfa; and GBM in adult patients whose cancer has progressed after prior treatment. Effectiveness based on tumor response, as no data have shown whether Avastin improves disease-related symptoms or survival in people previously treated for GBM
Approval conditionally granted in 2008 and withdrawn November 2011 for HER2- metastatic breast cancer (mBC) with Paclitaxel
Buparlisib (BKM120)
Sponsor: Novartis
Method of Action: Pan-PI3K inhibitor
Indications/Phase of Trial: mBC (Phase III and confirmatory Phase I/II); with Fulvestrant, in postmenopausal women with hormone receptor-positive HER2- locally advanced or mBC which progressed on or after aromatase inhibitor (AI) treatment (Phase III; BELLE-2 study recruiting as of November 2012); with Fulvestrant, in postmenopausal women with hormone receptor-positive HER2- AI-treated, locally-advanced or mBC who progressed on or after mTOR inhibitor-based treatment (Phase III; BELLE-3 study, recruiting as of October 2012); with Paclitaxel in patients with HER2- inoperable locally advanced or mBC, with or without PI3K pathway activation (Phase III; BELLE-4 study, recruiting as of November); metastatic castration-resistant prostate cancer (CRPC; Phase II; recruiting as of October); recurrent glioblastoma (Phase II; recruiting as of November); recurrent/metastatic head and neck squamous cell carcinoma (Phase II; recruiting as of October); endometrial cancer (Phase I/II); NSCLC (Phase I/II); prostate cancer (Phase I/II); GBM multiforme (Phase I/II); with Fulvestrant in postmenopausal women with estrogen receptor-positive metastatic breast cancer (Phase I); previously treated advanced colorectal cancer (Phase I)
Faslodex (Fulvestrant Injection)
Sponsor: AstraZeneca
Method of Action: Estrogen receptor antagonist
Indications/Phase of Trial: First line HR+ mBC (Phase III; FALCON study commenced Oct. 29)
Approved for HR+ mBC in women who have experienced menopause and whose breast cancer has worsened after they were treated with antiestrogen medications
Herceptin (Trastuzumab; RG597)
Sponsor: Roche, in partnership with Halozyme
Method of Action: Humanized monoclonal antibody designed to target and block the function of HER2+
Indications/Phase of Trial: EU: Early HER2+ breast cancer, subcutaneous formulation (Registration)
Approved for early-stage HER2+ breast cancer that has spread into the lymph nodes, and HER2+ breast cancer that has not spread into the lymph nodes and is estrogen receptor/progesterone receptor-negative (ER-/PR-) or have one high-risk feature. High-risk is defined as estrogen receptor/progesterone receptor-positive (ER+/PR+) with one of the following features: tumor size >2 cm, age <35 years, or tumor grade 2 or 3. Can be used with Adriamycin® (doxorubicin), Cytoxan® (cyclophosphamide), and either Taxol® (paclitaxel) or Taxotere® (docetaxel); or with Taxotere and Paraplatin® (carboplatin); or alone after treatment with multiple other therapies, including an anthracycline (Adriamycin)-based chemotherapy
Also approved alone for the treatment of HER2+ breast cancer in patients who have received one or more chemotherapy courses for metastatic disease; and with paclitaxel for first-line treatment of HER2+ mBC
Iniparib (Tivolza; BSI-201; SAR240550)
Sponsor: Sanofi, through acquisition of original developer BiPar Sciences
Method of Action: Poly (ADP-ribose) polymerase 1 (PARP1) inhibitor
Indications/Phase of Trial: Stage IV squamous NSCLC (Phase III; NME); solid tumors such as sarcoma and breast, uterine, lung, and ovarian cancers (Phase I/II)
Phase III trial in breast cancer failed January 2011 by failing to improve survival and progression-free survival (PFS) in breast cancer patients
Nexavar® (Sorafenib)
http://newdrugapprovals.wordpress.com/2013/07/16/nexavar-sorafenib/
Sponsor: Onyx Pharmaceuticals
Method of Action: Dual-action inhibitor that targets RAF/MEK/ERK pathway in tumor cells and tyrosine kinases
Indications/Phase of Trial: Liver cancer adjuvant (Phase III; STORM study); kidney cancer adjuvant (Phase III; SORCE/ASSURE study); thyroid cancer monotherapy (Phase III; DECISION study); breast cancer with capecitabine (Phase III; RESILIENCE study)
Approved for hepatocellular carcinoma (HCC) and RCC
Perjeta (Pertuzumab; RG1273)
Sponsor: Roche/Genentech
Method of Action: HER2/neu receptor antagonist
Indications/Phase of Trial: EU: With Herceptin and docetaxel chemotherapy for previously-untreated HER2+ mBC or locally recurrent, inoperable breast cancer in patients who have not received previous treatment or whose disease has returned after treatment in the early-stage setting (Registration)
U.S.: Approved June 2012 for HER2+ mBC with Herceptin (trastuzumab) and docetaxel, in patients who have not received prior anti-HER2 therapy or chemotherapy for metastatic disease
Switzerland: Approved August 2012 for HER2+ breast cancer with Herceptin (trastuzumab) and docetaxel in patients with advanced or locally recurring breast cancer that has not previously been treated with chemotherapy
Ridaforolimus (MK-8669; AP23573; formerly Deforolimus)
Sponsor: Merck, under exclusive worldwide license agreement with Ariad Pharmaceuticals
Method of Action: Oral inhibitor of mammalian target of rapamycin inhibitor (mTOR)
Indications/Phase of Trial: Maintenance therapy for metastatic soft-tissue sarcoma and bone sarcomas after at least four chemotherapy cycles (under review after receiving Complete Response letter from FDA in June; NME); breast cancer with exemestane, compared to breast cancer with dalotuzumab and exemestane (Phase II; recruiting as of November); advanced head and neck cancer, NSCLC and colon cancer, with cetuximab (Phase II); pediatric patients with advanced solid tumors (Phase I; recruiting as of September); with dalotuzumab in pediatric patients with advanced solid tumors (Phase I; recruiting as of August); advanced RCC, with vorinostat (Phase I; recruiting as of October 2012); breast cancer, with dalotuzumab (Phase I: recruiting as of September); endometrial and ovarian cancers, with paclitaxel and carboplatin (Phase I; recruiting as of September 2012); advanced cancer, with MK-2206 and MK-0752 (Phase I: recruiting as of September 2012); advanced cancer, with dalotuzumab, MK-2206 and MK-0752 (Phase I: recruiting as of August 2012)
Tivozanib (ASP4130; AV-951)
Sponsor: Aveo Oncology and Astellas
Method of Action: Tyrosine kinase inhibitor; inhibits VEGF receptor 1, 2, and 3
Indications/Phase of Trial: U.S.: Advanced RCC (Registration; NDA filed September 2012); tivozanib biomarkers in solid tumors (Phase II; BATON study); stage IV metastatic colorectal cancer (mCRC), with mFOLFOX6, and compared with bevacizumab and mFOLFOX6 (Phase II; recruiting as of November); additional data as first-line therapy for advanced RCC, followed by sunitinib (Phase II; TAURUS study, enrollment initiated in October 2012); advanced solid tumors, with capecitabine (Xeloda®; Phase I; recruiting as of October)
EU: Advanced RCC (Phase III)
Trastuzumab-DM1 (T-DM1; Trastuzumab emtansine; RG3502)
Sponsor: Roche, with linker technology developed by ImmunoGen
Method of Action: Antibody-drug conjugate, consisting of the antibody trastuzumab and the chemotherapy DM1 attached via a stable linker
Indications/Phase of Trial: U.S.: HER2+, unresectable locally-advanced or mBC who have received prior treatment with Herceptin (trastuzumab) and a taxane chemotherapy (Registration; Priority review approved Nov. 7; action date Feb. 26, 2013)
EU: Marketing Authorization Application for HER2+ mBC accepted for review by European Medicines Agency
Tyverb/Tykerb (lapatinib)
Sponsor: GlaxoSmithKline
Method of Action: Human epidermal growth factor receptor-2 (Her2) and epidermal growth factor receptor (EGFR) dual kinase inhibitor
Indications/Phase of Trial: mBC with trastuzumab (Registration); breast cancer, adjuvant therapy (Phase III); Gastric cancer (Phase III); head & neck squamous cell carcinoma, resectable disease (Phase III)
Xgeva (denosumab)
Sponsor: Amgen, with commercialization by GlaxoSmithKline in countries where Amgen has no presence
Method of Action: Fully human monoclonal antibody that specifically targets a ligand known as RANKL that binds to a receptor known as RANK
Indications/Phase of Trial: Delay or prevention of bone metastases in breast cancer (Phase III); delay or prevention of bone metastases in prostate cancer (Phase III)
Approved for prevention of fractures in men with advanced prostate cancer
Rejected in April for supplemental Biologics License Application to treat men with CRPC at high risk of developing bone metastases
Yondelis® (trabectedin)
Sponsor: Johnson & Johnson; developed in collaboration with PharmaMar
Method of Action: Binds to minor groove of DNA, interfering with the cell division and gene transcription processes, as well as DNA’s repair machinery
Indications/Phase of Trial: U.S.: Locally advanced or metastatic soft tissue sarcoma excluding leiomyosarcoma and liposarcoma who have relapsed or are refractory to standard-of-care treatment (Phase III; recruiting as of November); soft tissue sarcoma, excluding liposarcoma and leiomyosarcoma (L-type sarcoma), in previously-treated patients who cannot be expected to benefit from currently available therapeutic options (Phase III; recruiting as of November); locally advanced or metastatic L-sarcoma (liposarcoma or leiomyosarcoma) who were previously treated with at least an anthracycline and ifosfamide-containing regimen, or an anthracycline-containing regimen and one additional cytotoxic chemotherapy regimen, compared with dacarbazine group (Phase III; recruiting as of November); breast cancer and pediatric tumors (Phase II); Advanced malignancies and liver dysfunction (Phase I; recruiting as of November)
EU: Approved for advanced or metastatic soft tissue sarcoma, and for relapsed platinum-sensitive ovarian cancer, with DOXIL®/Caelyx®
Xtandi® Capsules (Enzalutamide; formerly MDV3100)
Sponsor: Medivation in collaboration with Astellas
Method of Action: Androgen receptor inhibitor
Indications/Phase of Trial: Prechemotherapy CRPC in patients who have failed luteinizing hormone-releasing hormone (LHRH) analog treatment only, as well as patients who have failed both LHRH analog and anti-androgen treatment. (Phase III; PREVAIL study); prostate cancer neoadjuvant therapy (Phase II); prechemo metastatic prostate cancer in Europe (Phase II; TERRAIN); prechemo metastatic and nonmetastatic prostate cancer patients in U.S. (Phase II; STRIVE); prostate cancer Hormone-naïve (Phase II; ASPIRE); prostate cancer with docetaxel (Phase I); breast cancer (Phase I)
EU: Marketing Authorization Application submitted June 2012 to European Medicines Agency, for patients with metastatic CRPC who have received docetaxel-based chemotherapy
Japan: Metastatic CRPC who have received docetaxel-based chemotherapy (Phase II)
Approved Aug. 31 for patients with metastatic CRPC who have previously received docetaxel. As a post-marketing requirement, Medivation and Astellas agreed to conduct an open-label safety study of Xtandi (160 mg/day) in patients at high risk for seizure, with data to be submitted to FDA in 2019



http://www.chemistryviews.org/details/ezine/5392681/Amide_Hydrogenation_in_Flow_Reactor.html
Amide Hydrogenation in Flow Reactor (wiley)
Amines are produced by amide hydrogenation over a bimetallic platinum–rhenium catalyst in a high-throughput vertical flow reactor
]]> A library of novel 5-nitroimidazole antibiotics displayed broad-spectrum activity
A library of novel 5-nitroimidazole antibiotics displayed broad-spectrum activity
The N-methylation of electron-deficient pyrroles was affected using dimethyl carbonate in the presence of DMF and catalytic DABCO. This alkylation methodology has proven useful for the alkylation of a variety of pyrroles in 72−98% yields and is considered to be greenchemistry relative to the more common use of methyl halides or dimethyl sulfate.

The use of alternative reaction solvents is reviewed in terms of life cycle. Supercritical CO2, ionic liquids, fluorous solvents, water, and renewable organics are compared on the basis of their solvency, ease of use, reusability, health and safety, environmental impact, and economic cost.
James H. Clark * and Stewart J. Tavener
Green Chemistry Centre, Department of Chemistry, University of York, Heslington, York, U.K. YO10 5DD
Org. Process Res. Dev., 2007, 11 (1), pp 149–155
DOI: 10.1021/op060160g
Publication Date (Web): November 4, 2006
http://pubs.acs.org/doi/full/10.1021/op060160g?prevSearch=GREEN%2BSOLVENTS&searchHistoryKey=
This article critically reviewS the use of alternative solvents in chemistry. Rather than follow the well-trodden path of discussing in turn the reactions that have been performed in each major type of alternative solvent, we will instead structure our article in terms of what we consider to be the fundamental issues: life cycle analysis (so as to establish the “green” and sustainability aspects from the outset), solvency (so as to consider what is needed in the application and how the alternatives manage to meet these needs), and application (to consider practical issues in both process and product).
]]>
Pariprazole sodium;Rabeprazole sodium;LY-307640;E-3810;Aciphex;Pariet
Rabeprazole /ˌræ.ˈbɛp.ræ.zɔːl/ is an antiulcer drug in the class of proton pump inhibitors. It was developed by Eisai Co. and is marketed by Janssen-Cilag as the sodium salt under the brand names AcipHex (/ˈæsɨfɛks/, referring to pH) in the US, Pariet in Europe, Brazil, Canada, Japan, Russia and Australia, Acigard, Cyra, Rabium, Esoon,Orporo, Parit, Rabemac, Rabiloz, Razo, Rabifast, Rablet and Rabsiv in India, and Zechin in Pakistan.
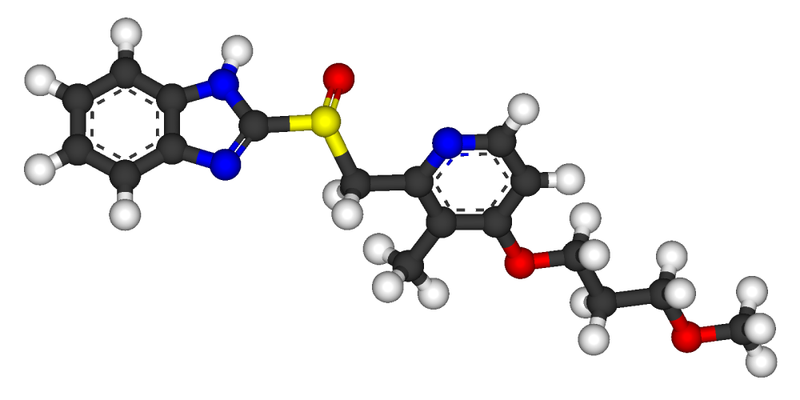
Rabeprazole, 2-[[[4-(3-Methoxypropoxy)-3-methyl-2-pyridinyl]methyl]sulfinyl]-1H-benzimidazole has the following structural formula
Rabeprazole belongs to a class of antisecretory compounds (substituted benzimidazole proton-pump inhibitors) that do not exhibit anticholinergic or histamine H2-receptor antagonist properties, but suppress gastric acid secretion by inhibiting the gastric H+, K+ATPase at the secretory surface of the gastric parietal cell. Because this enzyme is regarded as the acid (proton) pump within the parietal cell, rabeprazole has been characterized as a gastric proton-pump inhibitor. Rabeprazole blocks the final step of gastric acid secretion. So that it can effectively inhibit the secretion of an acid and is therefore effective in the therapy or prevention of human and animal peptic ulcer.
- US 5045552 discloses the preparation of Rabeprazole sodium by known traditional procedures, such as dissolution of the product in a mixture of stoichiometric quantity of aqueous sodium hydroxide and ethanol, then removal of water azeotropically, thereafter drying the residue at low pressure and then crystallization of the residue with less polar solvent such as diethyl ether, tert-butyl methyl ether.
The U.S. Pat. No. 5,045,552 discloses the Rabeprazole and many other substituted benzimidazole-type compounds having anti-ulcer activity. This patent further discloses the process for preparation of Rabeprazole by oxidation of Rabeprazole sulfide using 85% m-chloroperbenzoic acid in a mixture of dichloromethane and diethyl ether followed by work up to get product as oil. The obtained oil is crystallized from a mixture of dichloromethane/ether. Optionally the oily crude is dissolved in aqueous solution of sodium hydroxide. The obtained solution is subjected to azeotropic distillation with ethanol to remove water and adding ether to get crystalline Rabeprazole base.
According to the prior art, Rabeprazole base is crystallized using dichloromethane/ether to obtain crystalline off white product. The HPLC purity is less than or equal to 99% and the isolation procedure involves azeotropic distillation of water, during which the product is exposed to high temperature and leads to certain impurities. Repeated crystallization is needed to remove impurities to get desired quality. Using large volumes of chlorinated solvents in the plant leads to environmental hazardous.
Japanese patent application JP2001039975 teaches that the product obtained by example 33 of U.S. Pat. No. 5,045,552 with a melting range of 140-141° C. corresponds to amorphous rabeprazole sodium
The U.S. Pat. No. 6,919,459 patent also discloses the process for the preparation of Rabeprazole by oxidation of Rabeprazole sulfide using m-Chloroperbenzoic acid (m-CPBA) in a suitable solvent. The reaction mass is subjected to repeated washings at different pH levels and isolate the product from aqueous layer.
Rabeprazole is not stable at acidic conditions and decomposes to form unknown impurities. To remove these impurities repeated crystallizations are required to get desire quality of the final product.
The WO2006/117802 PCT application discloses the process for the preparation of Rabeprazole sodium by oxidation of Rabeprazole sulfide with sodium hypo halite solution in water or a mixture of water and water miscible solvent medium using alkali metal hydroxide and catalyst. The reaction mass is saturated by inorganic saturating agents and the Rabeprazole sodium salt is extracted with water immiscible organic solvent. Organic solvent is distilled and the residue is dissolved in second organic solvent to get clear solution, which is precipitated by adding antisolvent.
The WO2006/120701 PCT application discloses process for manufacture of amorphous Rabeprazole sodium by the reaction of Rabeprazole base with aqueous sodium hydroxide. Ethanol is added to the obtained solution. Solvents are distilled from the solution to get thick mass. Organic solvent is added to the obtained residue to get clear solution, to which antisolvent is added to get amorphous Rabeprazole sodium.
The prior art methods cited above have many disadvantages, these methods involve more number of organic solvents and lack successive extractions and washings of the layers during work up procedure. It leads to many impurities that ultimately affect on purity and yield loss of final product.
The U.S. Pat. No. 6,180,652 and WO 2003101452 PCT application discloses the process for the preparation of amorphous rabeprazole sodium, which is obtained by lyophilization of an aqueous solution of rabeprazole sodium acetone complex and an aqueous NaOH solution of Rabeprazole respectively.
Lyophilization technique is not suitable for production at industrial scale and it needs more time cycle and involves the cost.
We observed that rabeprazole is rapidly degraded in chlorinated solvent like dichloromethane to form unknown impurities, due to impurities while distillation gummy material is formed. It leads to yellowish color in final product, finally it leads to yield loss in final product.
According to prior art methods,
-
- (a) Dichloromethane/ether is used for final crystallization gives off white product with HPLC purity less than or equal to 99% and
- (b) Rabeprazole sodium is isolated by using azeotropic distillation. It needs high temperature to remove water and the reaction mass is exposed to high temperature to form unknown impurities, to remove these impurities repeated crystallizations are required to get desire quality of the final product
US 6,313,303 discloses the preparation of sulfoxides by oxidizing thio ether with a peroxoborate salt in the presence of an acid anhydride or a metal catalyst; and the preparation of sulfoxides by oxidizing thio ether with an N- halosuccinimide, l,3-dihalo-5,5-dimethyl-hydantoin or dichloroisocyanuric acid salt in the presence of a base.
IN 192030 discloses the purification process of Rabeprazole, in which sulfone enriched Rabeprazole is treated with an amino alcohol e.g. ethanolamine in the presence of an organic solvent, further the reaction mixture washed with water to remove the sulfone impurities. US 7,439,367 (IN218648, 058/MUM/2003, 193/MUM/2003) discloses the preparation of Rabeprazole by oxidizing its corresponding sulfide compound, where aqueous hypohalite solution is used as an oxidizing agent. The said oxidation is carried out at a controlled temperature and pH. During said oxidation the pH of the reaction mixture is maintained in the range of 9 to 12. This process utilizes catalyst such as pyridine, di-isopropyl ethyl amine and N,N-dimethyl amino pyridine.
US 7,060,837 discloses the purification of lansoprazole using ammonia, ammonium hydroxide, diethylamine, triethylamine and methylamine in the presence of solvent. The said patent utilizes acid for the isolation of lanzoprazole in pure form.
US 2008/0161579 (IN190/MUM/2005) discloses a process for the preparation of Rabeprazole sodium comprising oxidation of Rabeprazole sulfide with sodium hypohalite in water or a mixture of water and water miscible solvent using alkali metal hydroxide and catalyst. It also discloses a process for the preparation of Rabeprazole sulfide.
WO 2008/045777 (1856/CHE/2006) discloses the preparation of
Rabeprazole by oxidizing the corresponding sulfide compound using about 0.8 to 1.25 equivalents of an oxidizing agent in the presence of less than or about 2.25 equivalents of a base where aqueous sodium hypohalite used as an oxidizing agent.
WO 2006/024890 discloses a process for the preparation of Rabeprazole in which the Rabeprazole obtained was treated with the triethylamine in hexane. The use of n-hexane in the final stage is not suitable for manufacturing point of view as it is difficult to remove residual hexane solvent. There are several disadvantages associated with such known processes; all the methods reported in these prior arts leads to the formation of many impurities which ultimately affects the purity of the final product.
US 5,045,552 patent discloses the preparation of Rabeprazole by oxidizing the Rabeprazole sulfide using m-chloroperbenzoic acid as shown in scheme-I. The crude Rabeprazole was dissolved in sodium hydroxide and the resulting solution was azeotropically distilled together with ethanol thrice to remove the water. Finally ether was added to get the crystals of Rabeprazole sodium
WO 03/101452 discloses a method for the preparation of Rabeprazole sodium comprising dissolving Rabeprazole base in aqueous sodium hydroxide and then subjecting to lyophilization.



Souda, S.; Ueda, N.; Miyazawa, S.; Tagami, K.; Nomoto, S.; Okita, M.; Shimomura, N.; Kaneko, T.; Fujimoto, M.; Murakami, M.; Oketani, K.; Fujisaki, H.; Shibata, H.; Wakabayashi, T. (Eisai Co., Ltd.); Pyridine derivs., pharmaceutical compsns. comprising the same, the use of the same for the manufacture of medicaments having therapeutic or preventative value, and a process for preparing the same. AU 8781138; EP 0268956; EP 0475456; EP 0654471; EP 0786461; JP 1989006270; JP 1993247035; JP 1995291967; US 5045552; US 5998445 .
Castaner, J.; Prous, J.; E-3810. Drugs Fut 1991, 16, 1, 19.
Sohda, S.; Tagami, K.; Chiku, S.; Synthesis of 14C-labelled sodium pariprazole (E3810). J Label Compd Radiopharm 1993, 33, 9, 849.

Rabeprazole as “CYRA” (Systopic Labs Pvt Ltd), “Elpizole” (Orchid Chemicals & Pharmaceuticals), Elpizole-20 (Orchid Chemicals & Pharmaceuticals), Rablet (Lupin), Acigard (3D), AcipHex, Rabeloc, Pariet, Rabider (Duta Formulations) Rabsiv 20 (Saharsh Biologicals) is supplied in:
- Tablet, enteric-coated; 10 mg
- Tablet, enteric-coated; 20 mg
- Pali-Schöll I, Jensen-Jarolim E (April 2011). “Anti-acid medication as a risk factor for food allergy”. Allergy 66 (4): 469–77. doi:10.1111/j.1398-9995.2010.02511.x. PMID 21121928.
HPLC METHOD
Rabeprazole with more impurities, particularly at 2.12 RRT (393 mass), 3.51 RRT (491 mass), 4.47 RRT (457 mass), 4.85 RRT (684 mass) and 4.54 RRT (893 mass). The mass (molecular or formula weight) number of the impurities were identified using LCMS. Particularly, the obtained product contains unknown impurities of higher molecular weight in the range of 0.1-1.0 % at relative retention time (RRT) of 2.12, 3.51, 4.47, 4.85, and 4.54 RRT as measured by high performance liquid chromatography (HPLC) method provided below.
The purity of the product obtained is determined by high performance liquid chromatography method under the conditions mentioned below.
Column: Prontosil Kromabond 100-5-C18 (250 x 4.6 mm), 5μ,
Mobile phase A: 1.36g KH2PO4 to 1 litre water, 0.5ml OfEt3N, Mobile phase B: Methanol: ACN (95:5),
Diluent: Mobile phase A and ACN (70:30),
Flow Rate: 1.0 mL/min,
Detection: UV at 280 nm,
Injection Volume: 20 μL, Run Time: 60 min.
Column oven temperature: 3O0C. Surprisingly the applicant identified a method in which, crude Rabeprazole was treated with diethylamine and optionally addition of TBAB (tetrabutylammmonium bromide) as catalyst, where the impurity level reduced. Though the reported amines like triethyl amine, ethanolamine, and ammonia are effectively used to minimize sulfone impurity, those are failed or unsatisfactory to remove the impurities at 2.12 RRT, 3.51 RRT, 4.47 RRT, 4.85 RRT and 4.54 RRT.
SPECTRAL DATA
EP 1869015 B1 FOR RABEPRAZOLE SODIUM
IR Spectra (KBr, cm-1): 3382, 2927, 1583, 1462, 1384, 1298, 1269, 1190, 1157, 1093, 1018, 745.
H NMR Spectra [200 M Hz, CD3OD] δ (ppm): 8.23 – 8.25 (1H, d, ArH); 7.57 – 7.62 (2H, m, ArH); 7.0 – 7.09 (2H, m, ArH); 6.87 – 6.90 (1H, d, ArH); 4.57 – 4.63 (2H, d, O=S-CH2-Ar); 4.0 – 4.1 (2H, t, -O-CH2-CH2-); 3.49 – 3.55 (2H, t, -CH2-O-CH3); 3.31 (3H, s, -OCH3); 2.1 (3H, s, Ar-CH3); 1.96 – 2.0 (2H, t, -CH2-CH2-CH2-).
MP
As per the process described and exemplified in the U. S. Patent No.
5,045,552, rabeprazole sodium is prepared by oxidizing 2-[[4-(3- methoxyporpoxy)-3-methylpyridine-2-yl]rnethylthio]-1 H-benzimidazole with m- chloroperbenzoic acid to afford the rabeprazole base which is further converted to its sodium salt by using 0.1 N aqueous solution of sodium hydroxide, followed by addition of ethanol. The water is removed by azeotropic distillation and the product is precipitated by using ether as solvent such as diethyl ether, tert-butyl methyl ether. The melting point of the disclosed rabeprazole sodium salt is 140- 1410C. The isolation process described in the U. S. Patent No. 5,045,552 has numerous disadvantages such as large volume of solvents is required for azeotropic removal of water during which the product is exposed to high temperature and leads to certain impurities. Based on these drawbacks the isolation process finds to be unsuitable for preparation of amorphous rabeprazole sodium at commercial scale operations.
Japanese patent application JP 2001039975 indicates that the product obtained by example 33 of the U. S. Patent No. 5,045,552 with a melting point of
140-1410C corresponds to amorphous rabeprazole sodium. In this application, the X-ray powder diffraction pattern of the amorphous rabeprazole sodium is shown.
The PCT patent publication No. WO 03/101452 discloses a method for the preparation of rabeprazole sodium comprising dissolving rabeprazole base in aqueous sodium hydroxide and then subjecting to lyophilization. U.S. Patent No. 6,180,652 B1 (the ‘652 patent) describes acetone complex of rabeprazole sodium, process for its production and characterizes it by powder X-ray diffraction, infra-red spectroscopy and 1H-NMR spectroscopy. The ‘652 patent further reports a process for preparation of amorphous rabeprazole sodium by lyophilizing (freeze-drying) an aqueous solution of rabeprazole sodium acetone complex.
However, lyophilization is a technique, which is not suitable for production at industrial scale because this process presents serious limitations on cost, time, equipment capability and environmental protection.
According to PCT patent publication No. WO 2004/085424A1 , amorphous rabeprazole sodium is obtained by heating the rabeprazole sodium acetone complex at elevated temperature, preferably between 100 and 1100C. It is well known that exposing rabeprazole-type compounds to high temperatures increases the risk of decomposition to form impurities and as such, heat treatment of rabeprazole sodium acetone complex into amorphous rabeprazole sodium is not adequate for the production of a rabeprazole which is suitable for pharmaceutical use.
PCT patent publication No. WO 2007/023393 A2 reports a process for preparation of amorphous rabeprazole sodium, the said process comprises: i) contacting rabeprazole sodium acetone complex with a first solvent system which includes a hydrocarbon solvent or an ether solvent or an alcohol solvent or mixtures thereof; ii) filtering the solid from the solvent system used in step i) or distilling the solvent system used in step i) under reduced or atmospheric pressure, to thereby obtain a residue; iii) contacting the wet solid or the residue of step ii) with a second solvent system which includes a hydrocarbon solvent or an ether solvent; and iv) filtering to obtain a wet solid from the solvent system used in step iii) to obtain a wet solid.
The methods for preparation of amorphous rabeprazole sodium as described in the patents U.S. Patent No. 6,180,652 B1 , PCT patent publication No. WO 2004/085424A1 and PCT patent publication No. WO 2007/023393 A2 involves lengthy process i.e., proceeds via rabeprazole sodium acetone complex intermediate and also the yields obtained in these processes are very low.
U.S. Patent Application No. US2004/0180935A1 teaches a process for production of amorphous rabeprazole sodium by dissolving rabeprazole acid in a mixture of sodium hydroxide and methanol at 25-350C, removing the solvent by evaporation and precipitating the product by adding petroleum ether.
PCT patent publication No. WO 2006/120701 A1 teaches a process for manufacture of amorphous rabeprazole sodium with mean particle diameter between 10 to 55 μm, the said process comprises, addition of rabeprazole to aqueous sodium hydroxide; addition of ethyl alcohol to the solution; distillation of solvents from the solution thus obtained till thick mass is obtained; addition of an organic solvent selected from ethyl acetate, dichloromethane, chloroform, butyl acetate, ethanol, isopropyl alcohol, methanol, tetrahydrofuran, to the residue to obtain a clear solution; addition of this clear solution to an anti-solvent includes diisopropyl ether, diethyl ether, methyl tert-butyl ether, under agitation and isolation of the product.
Since a solvent may play an important role in increasing the yield rate or in determination of physical properties of drug substance such as crystal form, purity, solubility, etc., even if such a solvent is known to be toxic, there may be many cases that the use thereof in the preparation of drug substance cannot be avoided in terms of risk benefits. In such cases, this guideline (ICH guidelines Q3C(R3)) decrees that a concentration of a residual solvent in drug substance should be not more than a specified value, which is toxicologically acceptable. The methods for preparation of amorphous rabeprazole sodium as described in the patents, U.S. Patent Application No. US2004/0180935A1 and PCT patent publication No. WO 2006/120701 A1 suffers with residual solvent problem and thereby commercially not viable. These methods utilize the solvents like diisopropyl ether and petroleum ether as precipitating solvents. These solvents are difficult to remove completely by practical manufacturing techniques. According to the ICH guidelines Q3C(R3), there is no adequate toxicological data for the solvents like diisopropyl ether and petroleum ether on which to base a PDE was found. However, a need still remains for an improved and commercially viable process of preparing pure amorphous rabeprazole sodium that would solve the aforesaid problems associated with processes described in the prior art, which will be suitable for largr-scale preparation, in terms of simplicity, chemical yield and purity of the product, and which would carry out with comparatively smaller volume of solvent
]]>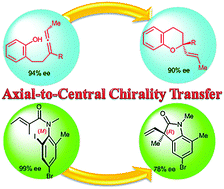
Axial-to-central chirality transfer in cyclization processes
Substrates, bearing axial chirality, can cyclize intra- or inter-molecularly with concomitant transfer of axial-to-central chirality to produce at least one stereocenter. In order to satisfy a strict definition of axial-to-central chirality transfer, the initial axial chirality must be lost during the cyclization process. Highly functionalized enantiopure carbocycles and heterocycles were prepared using this strategy. The transformations of configurationally stable substrates take place with high regio- and stereo-selectivity. Selected examples involving allenes, biaryls, arylamides and transient axially chiral short-lived species are discussed. Special attention is focused on the mechanistic rationale of the chirality transfer.
DOI: 10.1039/C3CS60182J
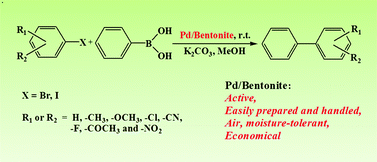
DOI: 10.1039/C3GC41469H, Paper
The Pd/bentonite catalyst prepared by a simple impregnation method in water is very active and stable for the Suzuki-Miyaura reaction.

Once the heteronuclear seeds had been used in the lab, the cocrystal formed regardless of whether or not the seeds were used
The caffeine•benzoic acid cocrystal that has eluded scientists for 60 years has finally been crystallised
Cocrystals are crystalline materials composed of two or more molecules held together within the same crystal lattice. Cocrystallisation is significant in the pharmaceutical industry, where drug molecules are screened for cocrystal formation in order to improve their solubility, stability and bioavailability. This has the added advantage of increasing the number of crystal forms that can be considered for drug formulation while simultaneously maximising patent protection.