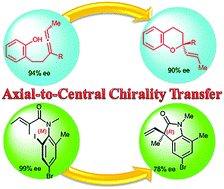
Axial-to-central chirality transfer in cyclization processes
Substrates, bearing axial chirality, can cyclize intra- or inter-molecularly with concomitant transfer of axial-to-central chirality to produce at least one stereocenter. In order to satisfy a strict definition of axial-to-central chirality transfer, the initial axial chirality must be lost during the cyclization process. Highly functionalized enantiopure carbocycles and heterocycles were prepared using this strategy. The transformations of configurationally stable substrates take place with high regio- and stereo-selectivity. Selected examples involving allenes, biaryls, arylamides and transient axially chiral short-lived species are discussed. Special attention is focused on the mechanistic rationale of the chirality transfer.
DOI: 10.1039/C3CS60182J
DOI: 10.1039/C3GC41469H, Paper
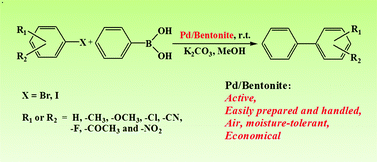
The Pd/bentonite catalyst prepared by a simple impregnation method in water is very active and stable for the Suzuki-Miyaura reaction.

Once the heteronuclear seeds had been used in the lab, the cocrystal formed regardless of whether or not the seeds were used
The caffeine•benzoic acid cocrystal that has eluded scientists for 60 years has finally been crystallised
Cocrystals are crystalline materials composed of two or more molecules held together within the same crystal lattice. Cocrystallisation is significant in the pharmaceutical industry, where drug molecules are screened for cocrystal formation in order to improve their solubility, stability and bioavailability. This has the added advantage of increasing the number of crystal forms that can be considered for drug formulation while simultaneously maximising patent protection.

The molecule of about 2 nm in size is kept stable between two metal electrodes for several days. Image: Christian Grupe/KIT
A team of physicists has succeeded in performing an extraordinary experiment: They demonstrated how magnetism that generally manifests itself by a force between two magnetized objects acts within a single molecule. This discovery is of high significance to fundamental research and provides scientists with a new tool to better understand magnetism as an elementary phenomenon of physics. The researchers published their results in the latest issue of Nature Nanotechnology.
The smallest unit of a magnet is the magnetic moment of a single atom or ion. Researchers in Germany wanted to find out whether the magnetism of a pair of magnetic moments can be measured electrically in a single molecule. To do this, they succeeded in performing an extraordinary experiment which shows how magnetism that generally manifests itself by a force between two magnetized objects acts within a single molecule.
]]>
This model shows the role of vesicles, vesicle chains and membrane tubes in M. xanthus biofilms. The scientists believe these connections help cells exchange signals and material. Image: Auer laboratory
Using several imaging techniques, Lawrence Berkeley National Laboratory scientists found that a common soil bacterium stays connected by a network of chain-like membranes. They believe the bacterium uses its network to coordinate social activities-such as evading bacterial enemies and snaring prey-without revealing its location. Even bacteria use social networks
read at
]]>allow slideshare to load………
]]>

 Winstein proposed the nonclassical structure of the 2-norbornyl cation, now shown to be correct
Winstein proposed the nonclassical structure of the 2-norbornyl cation, now shown to be correct
DOI: 10.1039/C3GC40587G, Communication
Visible-light mediated heterogeneous C-H functionalization: oxidative multi-component reactions using a recyclable titanium dioxide (TiO2) catalyst
Visible-light mediated heterogeneous C–H functionalization of tertiary amines provides access to a variety of α-amino amides. An oxidative, titanium dioxide catalyzed, Ugi-type, three-component reaction has been developed in which the catalyst can be recycled without loss of activity.

This model shows the five-sided cyanostar macrocycle capturing perchlorate at its center. (Credit: Amar Flood)], Chemists at Indiana University Bloomington have created a symmetrical, five-sided macrocycle that is easy to synthesize and has characteristics that may help expand the molecular tool box available to researchers in biology, chemistry and materials sciences.The molecule, which the researchers call cyanostar, was developed in the lab of Amar Flood, associate professor in the Department of Chemistry in the College of Arts and Sciences. It is described in an article in the journal Nature Chemistry, scheduled for publication in August and available online. Semin Lee, Chun-Hsing Chen, Amar H. Flood. A pentagonal cyanostar macrocycle with cyanostilbene CH donors binds anions and forms dialkylphosphate [3]rotaxanes. Nature Chemistry, 2013; DOI: 10.1038/nchem.1668
click link
]]>