Damiana contains a glycoside compound called arbutin. In the urinary tract, arbutin is converted into a chemical called hydroquinone. In large amounts, hydroquinone can cause nausea, vomiting, tinnitus (ringing in the ears, convulsions, and eventually, collapse and death.

In the United States, the Food and Drug Administration (FDA) has approved pregabalin for adjunctive therapy for adults with partial onset seizures, management of postherpetic neuralgia andneuropathic pain associated with spinal cord injury and diabetic peripheral neuropathy, and the treatment of fibromyalgia. Pregabalin has also been approved in the European Union and Russia(but not in US) for treatment of generalized anxiety disorder.
READ AT ……………http://www.rsc.org/chemistryworld/News/2008/July/09070801.asp

-
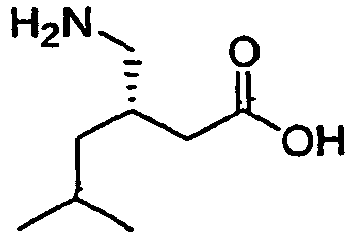
(S)-Pregabalin, (S)-(+)-3-(aminomethyl)-5-methylhexanoic acid, a compound having the chemical structure,
is also known as γ-amino butyric acid or (S)-3-isobutyl GABA. (S)-Pregabalin, marketed under the name LYRICA®, has been found to activate GAD (L-glutamic acid decarboxylase). (S)-Pregabalin has a dose dependent protective effect on-seizure, and is a CNS-active compound. (S)-Pregabalin is useful in anticonvulsant therapy, due to its activation of GAD, promoting the production of GABA, one of the brain’s major inhibitory neurotransmitters, which is released at 30 percent of the brains synapses. (S)-Pregabalin has analgesic, anticonvulsant, and anxiolytic activity.
-
[0003]
-
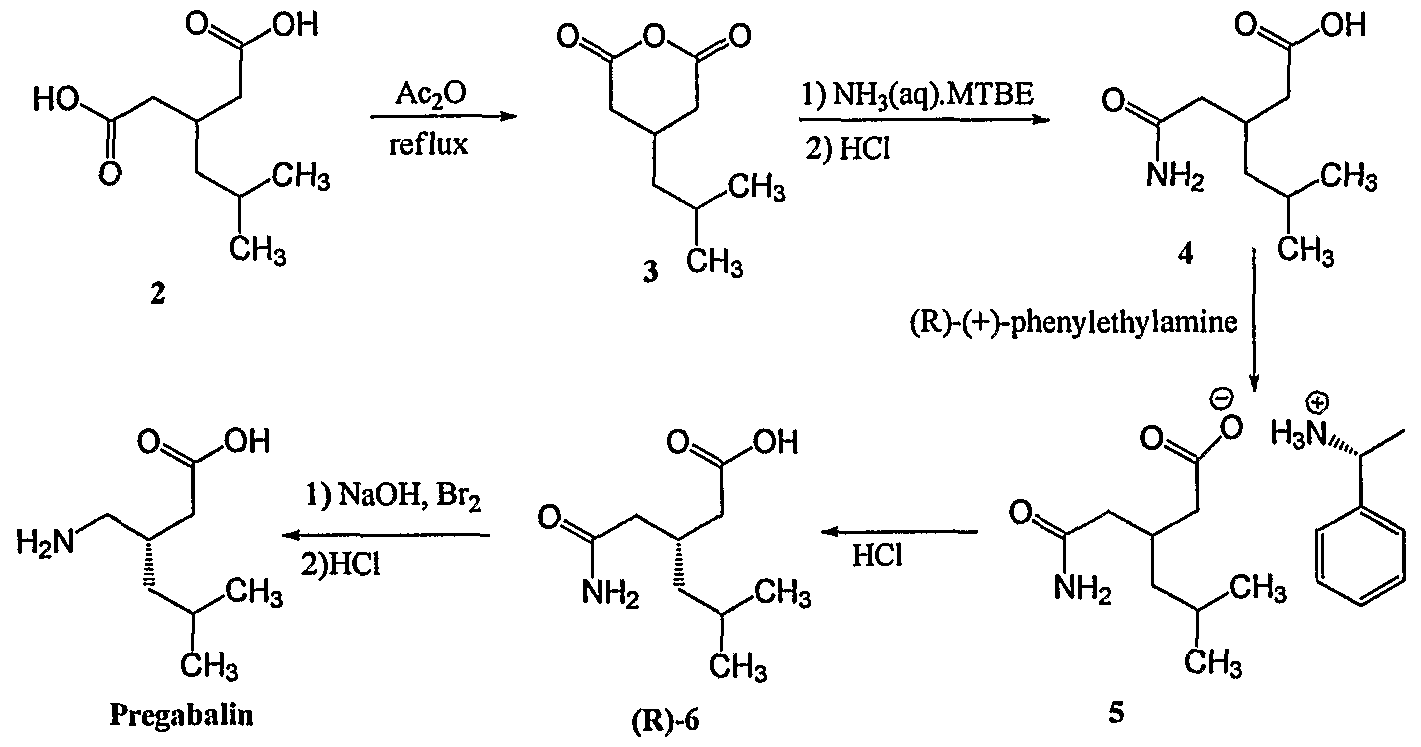
[0004]In Scheme 1, 3-isobutyl glutaric acid, compound 2, is converted into the corresponding anhydride, compound 3, by treatment with refluxing acetic anhydride. The reaction of the anhydride with NH4OH produces the glutaric acid mono-amide, compound 4, which is resolved with (R)-1-phenylethylamine, yielding the (R)-phenylethylamine salt of (R)-3-(carbamoylmethyl)-5-methylhexanoic acid, compound 5. Combining the salt with an acid liberates the R enantiomer, compound 6. Finally, a Hoffmann degradation with Br2/NaOH provides (S)-Pregabalin. A disadvantage of this method is that it requires separating the two enantiomers, thereby resulting in the loss of half the product, such that the process cost is high.
-
[0005]Several stereoselective processes for the synthesis of (S)-Pregabalin have been disclosed. For example, U.S. Patent No. 5,599,973 discloses the preparation of (S)-Pregabalin using stoichiometric (+)-4-methyl-5-phenyl-2-oxazolidinone as a chiral auxiliary that may be recycled. In general, however, that route is of limited use for scale-up, principally due to the low temperature required for the reactions, the use of pyrophoric reagent, such as, butyl lithium, to side reactions, and due to a low overall yield.
-
[0006]Another process is disclosed inU.S. Patent Application Publication No. 2003/0212290 , which discloses asymmetric hydrogenation of a cyano-substituted olefin, compound 7, to produce a cyano precursor of (S)-3-(aminomethyl)-5-methyl hexanoic acid, compound 8, as seen in scheme 2.
-
[0007]Subsequent reduction of the nitrile in compound 8 by catalytic hydrogenation produces (S)-Pregabalin. The cyano hexenoate starting material, compound 7, is prepared from 2-methyl propanal and acrylonitrile (Yamamoto et al, Bull. Chem. Soc. Jap., 58, 3397 (1985)). However, the disclosed method requires carbon monoxide under high pressure, raising serious problems in adapting this scheme for production scale processes.
-
[0008]A process published by G.M. Sammis, et al., J. Am. Chem. Soc., 125(15), 4442-43 (2003), takes advantage of the asymmetric catalysis of cyanide conjugate addition reactions. The method discloses the application of aluminum salen catalysts to the conjugate addition of hydrogen cyanide to α,β-unsaturated imides as shown in scheme 3. Reportedly, TMSCN is a useful source of cyanide that can be used in the place of HCN. Although the reaction is highly selective, this process is not practicable for large scale production due to the use of highly poisonous reagents. Moreover, the last reductive step requires high pressure hydrogen, which only adds to the difficulties required for adapting this scheme for a production scale process.
-
[0009]In 1989, Silverman reported a convenient synthesis of 3-alkyl-4-amino acids compounds in SYNTHESIS, Vol. 12, 953-944 (1989). Using 2-alkenoic esters as a substrate, a series of GABA analogs were produced by Michael addition of nitromethane to α,β-unsaturated compounds, followed by hydrogenation at atmospheric pressure of the nitro compound to amine moiety as depicted in scheme 4.
-
[0010]Further resolution of compound 14 may be employed to resolve Pregabalin. This, of course, results in the loss of 50 percent of the product, a serious disadvantage. However, the disclosed methodology reveals that the nitro compound can serve as an intermediate for the synthesis of 3-alkyl-4-amino acids.
-
[0011]Recent studies have indicated that cinchona alkaloids are broadly effective in chiral organic chemistry. A range of nitroalkenes were reportedly treated with dimethyl or diethyl malonate in THF in the presence of cinchona alkaloids to provide high enantiomeric selectivity of compound 15,
and its analogues. For example, see H. Li, et al., J. Am. Chem. Soc, 126(32), 9906-07 (2004). These catalysts are easily accessible from either quinine or quinidine, and are reportedly highly efficient for a synthetically C-C bond forming asymmetric conjugate addition as shown in scheme 5.
-
[0012]R3 represents several alkyl and aryl groups. The scope of the reaction has been extended to other nitroolefins and applied to prepare ABT-546 employing bis(oxazoline)Mg(OTf)2. See, for example, D.M. Barnes, et al., J. Am. Chem. Soc., 124(44), 13097-13105 (2002).
-
[0013]Other groups have investigated a new class of bifunctional catalysts bearing a thiourea moiety and an amino group on a chiral scaffold. SeeT. Okino, et al., J. Am. Chem. Soc., 127(1), 119-125 (2005). On the basis of a catalytic Michael addition to the nitroolefin with enantiomeric selectivity, they were able to prepare a series of analogues of compound 15.
-
[0014]Thus, there is a need in the art for new processes for the preparation of (S)-Pregabalin that does not suffer from the disadvantages mentioned above. Chemical Abstracts, database accession no. 2005:236589 refers to a process for the synthesis of pregabalin using methyl cyanoacetate, by condensation, addition, cyclization, aminolysis, Hoffmann rearrangement and resolution with (S)-mandelic acid.
Karenewsky, D. S., et al., J. Org. Chem., 1991, 56, 3744-3747, discloses reaction of a glutaric acid anhydride with (S)-1-phenyethylamine to prepare the corresponding amide, which is subsequently used to prepare β-ketophosphonate derivatives.
Verma, R., et al., J. Chem. Soc. Perkin Trans. I, 1999, 257-264, discloses desymmetrization of prochiral anhydrides with Evans’ oxazolidinones to prepare homochiral glutaric and adipic acid derivatives.
Shintani, R. et al., Angew. Chem. Int. Ed. 2002, 41 (6), 1057-1059, discloses the desymmetrization of various glutaric acid anhydrides using Grignard reagents in the presence of (-)-sparteine
Highly Enantioselective Alkenylation of Cyclic α,β-Unsaturated Carbonyl Compounds as Catalyzed by a Rhodium–Diene Complex: Application to the Synthesis of (S)-Pregabalin and (–)-α-Kainic Acid.Chem. Eur. J. 2012;18: 13274-13278





fruits from Rosaceae family (Genus: Prunus). The collected fruits are peach (Prunus persica), Himalayan wild cherry (Prunus avium), Red Indian plum (Prunusdomestica), Himalayan plum (Prunus americana), apricot (Prunus armeniaca) and shakarpara (white apricot, a hybrid cultivar of normal apricot found in Nepal and India). All the six newly found HNLs are R-selective, i.e., they yield R-mandelonitrile from benzaldehyde bySi-facial attack of the cyanide anion. The enantioselectivity obtained for the formation of mandelonitriles by all the six HNLs are in the range of 60-93%. The best results are obtained with PavHNL and ParsHNL (both provide 93% ee), where as PpHNL is the least enantioselective (provides 60% ee for R-mandelonitrile formation). The main object of the project proposal will be the development of efficient biocatalytic route for various value added products such as Pregablin, Baclofen and Pril drugs

http://www.google.com/patents/EP2053040A1
http://www.google.com/patents/EP2170813A2

http://orgspectroscopyint.blogspot.in/2013/11/pregabalin-spectral-data.html
– See more at: http://worlddrugtracker.blogspot.in/#sthash.AUeFbwD7.dpuf
]]>
Pariprazole sodium;Rabeprazole sodium;LY-307640;E-3810;Aciphex;Pariet
Rabeprazole /ˌræ.ˈbɛp.ræ.zɔːl/ is an antiulcer drug in the class of proton pump inhibitors. It was developed by Eisai Co. and is marketed by Janssen-Cilag as the sodium salt under the brand names AcipHex (/ˈæsɨfɛks/, referring to pH) in the US, Pariet in Europe, Brazil, Canada, Japan, Russia and Australia, Acigard, Cyra, Rabium, Esoon,Orporo, Parit, Rabemac, Rabiloz, Razo, Rabifast, Rablet and Rabsiv in India, and Zechin in Pakistan.
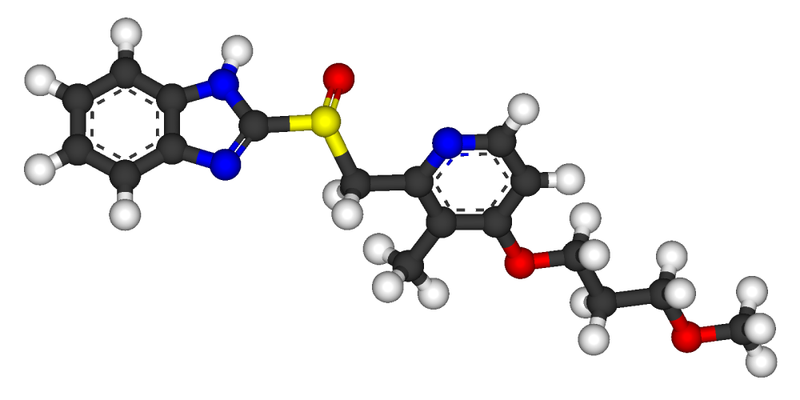
Rabeprazole, 2-[[[4-(3-Methoxypropoxy)-3-methyl-2-pyridinyl]methyl]sulfinyl]-1H-benzimidazole has the following structural formula
Rabeprazole belongs to a class of antisecretory compounds (substituted benzimidazole proton-pump inhibitors) that do not exhibit anticholinergic or histamine H2-receptor antagonist properties, but suppress gastric acid secretion by inhibiting the gastric H+, K+ATPase at the secretory surface of the gastric parietal cell. Because this enzyme is regarded as the acid (proton) pump within the parietal cell, rabeprazole has been characterized as a gastric proton-pump inhibitor. Rabeprazole blocks the final step of gastric acid secretion. So that it can effectively inhibit the secretion of an acid and is therefore effective in the therapy or prevention of human and animal peptic ulcer.
- US 5045552 discloses the preparation of Rabeprazole sodium by known traditional procedures, such as dissolution of the product in a mixture of stoichiometric quantity of aqueous sodium hydroxide and ethanol, then removal of water azeotropically, thereafter drying the residue at low pressure and then crystallization of the residue with less polar solvent such as diethyl ether, tert-butyl methyl ether.
The U.S. Pat. No. 5,045,552 discloses the Rabeprazole and many other substituted benzimidazole-type compounds having anti-ulcer activity. This patent further discloses the process for preparation of Rabeprazole by oxidation of Rabeprazole sulfide using 85% m-chloroperbenzoic acid in a mixture of dichloromethane and diethyl ether followed by work up to get product as oil. The obtained oil is crystallized from a mixture of dichloromethane/ether. Optionally the oily crude is dissolved in aqueous solution of sodium hydroxide. The obtained solution is subjected to azeotropic distillation with ethanol to remove water and adding ether to get crystalline Rabeprazole base.
According to the prior art, Rabeprazole base is crystallized using dichloromethane/ether to obtain crystalline off white product. The HPLC purity is less than or equal to 99% and the isolation procedure involves azeotropic distillation of water, during which the product is exposed to high temperature and leads to certain impurities. Repeated crystallization is needed to remove impurities to get desired quality. Using large volumes of chlorinated solvents in the plant leads to environmental hazardous.
Japanese patent application JP2001039975 teaches that the product obtained by example 33 of U.S. Pat. No. 5,045,552 with a melting range of 140-141° C. corresponds to amorphous rabeprazole sodium
The U.S. Pat. No. 6,919,459 patent also discloses the process for the preparation of Rabeprazole by oxidation of Rabeprazole sulfide using m-Chloroperbenzoic acid (m-CPBA) in a suitable solvent. The reaction mass is subjected to repeated washings at different pH levels and isolate the product from aqueous layer.
Rabeprazole is not stable at acidic conditions and decomposes to form unknown impurities. To remove these impurities repeated crystallizations are required to get desire quality of the final product.
The WO2006/117802 PCT application discloses the process for the preparation of Rabeprazole sodium by oxidation of Rabeprazole sulfide with sodium hypo halite solution in water or a mixture of water and water miscible solvent medium using alkali metal hydroxide and catalyst. The reaction mass is saturated by inorganic saturating agents and the Rabeprazole sodium salt is extracted with water immiscible organic solvent. Organic solvent is distilled and the residue is dissolved in second organic solvent to get clear solution, which is precipitated by adding antisolvent.
The WO2006/120701 PCT application discloses process for manufacture of amorphous Rabeprazole sodium by the reaction of Rabeprazole base with aqueous sodium hydroxide. Ethanol is added to the obtained solution. Solvents are distilled from the solution to get thick mass. Organic solvent is added to the obtained residue to get clear solution, to which antisolvent is added to get amorphous Rabeprazole sodium.
The prior art methods cited above have many disadvantages, these methods involve more number of organic solvents and lack successive extractions and washings of the layers during work up procedure. It leads to many impurities that ultimately affect on purity and yield loss of final product.
The U.S. Pat. No. 6,180,652 and WO 2003101452 PCT application discloses the process for the preparation of amorphous rabeprazole sodium, which is obtained by lyophilization of an aqueous solution of rabeprazole sodium acetone complex and an aqueous NaOH solution of Rabeprazole respectively.
Lyophilization technique is not suitable for production at industrial scale and it needs more time cycle and involves the cost.
We observed that rabeprazole is rapidly degraded in chlorinated solvent like dichloromethane to form unknown impurities, due to impurities while distillation gummy material is formed. It leads to yellowish color in final product, finally it leads to yield loss in final product.
According to prior art methods,
-
- (a) Dichloromethane/ether is used for final crystallization gives off white product with HPLC purity less than or equal to 99% and
- (b) Rabeprazole sodium is isolated by using azeotropic distillation. It needs high temperature to remove water and the reaction mass is exposed to high temperature to form unknown impurities, to remove these impurities repeated crystallizations are required to get desire quality of the final product
US 6,313,303 discloses the preparation of sulfoxides by oxidizing thio ether with a peroxoborate salt in the presence of an acid anhydride or a metal catalyst; and the preparation of sulfoxides by oxidizing thio ether with an N- halosuccinimide, l,3-dihalo-5,5-dimethyl-hydantoin or dichloroisocyanuric acid salt in the presence of a base.
IN 192030 discloses the purification process of Rabeprazole, in which sulfone enriched Rabeprazole is treated with an amino alcohol e.g. ethanolamine in the presence of an organic solvent, further the reaction mixture washed with water to remove the sulfone impurities. US 7,439,367 (IN218648, 058/MUM/2003, 193/MUM/2003) discloses the preparation of Rabeprazole by oxidizing its corresponding sulfide compound, where aqueous hypohalite solution is used as an oxidizing agent. The said oxidation is carried out at a controlled temperature and pH. During said oxidation the pH of the reaction mixture is maintained in the range of 9 to 12. This process utilizes catalyst such as pyridine, di-isopropyl ethyl amine and N,N-dimethyl amino pyridine.
US 7,060,837 discloses the purification of lansoprazole using ammonia, ammonium hydroxide, diethylamine, triethylamine and methylamine in the presence of solvent. The said patent utilizes acid for the isolation of lanzoprazole in pure form.
US 2008/0161579 (IN190/MUM/2005) discloses a process for the preparation of Rabeprazole sodium comprising oxidation of Rabeprazole sulfide with sodium hypohalite in water or a mixture of water and water miscible solvent using alkali metal hydroxide and catalyst. It also discloses a process for the preparation of Rabeprazole sulfide.
WO 2008/045777 (1856/CHE/2006) discloses the preparation of
Rabeprazole by oxidizing the corresponding sulfide compound using about 0.8 to 1.25 equivalents of an oxidizing agent in the presence of less than or about 2.25 equivalents of a base where aqueous sodium hypohalite used as an oxidizing agent.
WO 2006/024890 discloses a process for the preparation of Rabeprazole in which the Rabeprazole obtained was treated with the triethylamine in hexane. The use of n-hexane in the final stage is not suitable for manufacturing point of view as it is difficult to remove residual hexane solvent. There are several disadvantages associated with such known processes; all the methods reported in these prior arts leads to the formation of many impurities which ultimately affects the purity of the final product.
US 5,045,552 patent discloses the preparation of Rabeprazole by oxidizing the Rabeprazole sulfide using m-chloroperbenzoic acid as shown in scheme-I. The crude Rabeprazole was dissolved in sodium hydroxide and the resulting solution was azeotropically distilled together with ethanol thrice to remove the water. Finally ether was added to get the crystals of Rabeprazole sodium
WO 03/101452 discloses a method for the preparation of Rabeprazole sodium comprising dissolving Rabeprazole base in aqueous sodium hydroxide and then subjecting to lyophilization.



Souda, S.; Ueda, N.; Miyazawa, S.; Tagami, K.; Nomoto, S.; Okita, M.; Shimomura, N.; Kaneko, T.; Fujimoto, M.; Murakami, M.; Oketani, K.; Fujisaki, H.; Shibata, H.; Wakabayashi, T. (Eisai Co., Ltd.); Pyridine derivs., pharmaceutical compsns. comprising the same, the use of the same for the manufacture of medicaments having therapeutic or preventative value, and a process for preparing the same. AU 8781138; EP 0268956; EP 0475456; EP 0654471; EP 0786461; JP 1989006270; JP 1993247035; JP 1995291967; US 5045552; US 5998445 .
Castaner, J.; Prous, J.; E-3810. Drugs Fut 1991, 16, 1, 19.
Sohda, S.; Tagami, K.; Chiku, S.; Synthesis of 14C-labelled sodium pariprazole (E3810). J Label Compd Radiopharm 1993, 33, 9, 849.

Rabeprazole as “CYRA” (Systopic Labs Pvt Ltd), “Elpizole” (Orchid Chemicals & Pharmaceuticals), Elpizole-20 (Orchid Chemicals & Pharmaceuticals), Rablet (Lupin), Acigard (3D), AcipHex, Rabeloc, Pariet, Rabider (Duta Formulations) Rabsiv 20 (Saharsh Biologicals) is supplied in:
- Tablet, enteric-coated; 10 mg
- Tablet, enteric-coated; 20 mg
- Pali-Schöll I, Jensen-Jarolim E (April 2011). “Anti-acid medication as a risk factor for food allergy”. Allergy 66 (4): 469–77. doi:10.1111/j.1398-9995.2010.02511.x. PMID 21121928.
HPLC METHOD
Rabeprazole with more impurities, particularly at 2.12 RRT (393 mass), 3.51 RRT (491 mass), 4.47 RRT (457 mass), 4.85 RRT (684 mass) and 4.54 RRT (893 mass). The mass (molecular or formula weight) number of the impurities were identified using LCMS. Particularly, the obtained product contains unknown impurities of higher molecular weight in the range of 0.1-1.0 % at relative retention time (RRT) of 2.12, 3.51, 4.47, 4.85, and 4.54 RRT as measured by high performance liquid chromatography (HPLC) method provided below.
The purity of the product obtained is determined by high performance liquid chromatography method under the conditions mentioned below.
Column: Prontosil Kromabond 100-5-C18 (250 x 4.6 mm), 5μ,
Mobile phase A: 1.36g KH2PO4 to 1 litre water, 0.5ml OfEt3N, Mobile phase B: Methanol: ACN (95:5),
Diluent: Mobile phase A and ACN (70:30),
Flow Rate: 1.0 mL/min,
Detection: UV at 280 nm,
Injection Volume: 20 μL, Run Time: 60 min.
Column oven temperature: 3O0C. Surprisingly the applicant identified a method in which, crude Rabeprazole was treated with diethylamine and optionally addition of TBAB (tetrabutylammmonium bromide) as catalyst, where the impurity level reduced. Though the reported amines like triethyl amine, ethanolamine, and ammonia are effectively used to minimize sulfone impurity, those are failed or unsatisfactory to remove the impurities at 2.12 RRT, 3.51 RRT, 4.47 RRT, 4.85 RRT and 4.54 RRT.
SPECTRAL DATA
EP 1869015 B1 FOR RABEPRAZOLE SODIUM
IR Spectra (KBr, cm-1): 3382, 2927, 1583, 1462, 1384, 1298, 1269, 1190, 1157, 1093, 1018, 745.
H NMR Spectra [200 M Hz, CD3OD] δ (ppm): 8.23 – 8.25 (1H, d, ArH); 7.57 – 7.62 (2H, m, ArH); 7.0 – 7.09 (2H, m, ArH); 6.87 – 6.90 (1H, d, ArH); 4.57 – 4.63 (2H, d, O=S-CH2-Ar); 4.0 – 4.1 (2H, t, -O-CH2-CH2-); 3.49 – 3.55 (2H, t, -CH2-O-CH3); 3.31 (3H, s, -OCH3); 2.1 (3H, s, Ar-CH3); 1.96 – 2.0 (2H, t, -CH2-CH2-CH2-).
MP
As per the process described and exemplified in the U. S. Patent No.
5,045,552, rabeprazole sodium is prepared by oxidizing 2-[[4-(3- methoxyporpoxy)-3-methylpyridine-2-yl]rnethylthio]-1 H-benzimidazole with m- chloroperbenzoic acid to afford the rabeprazole base which is further converted to its sodium salt by using 0.1 N aqueous solution of sodium hydroxide, followed by addition of ethanol. The water is removed by azeotropic distillation and the product is precipitated by using ether as solvent such as diethyl ether, tert-butyl methyl ether. The melting point of the disclosed rabeprazole sodium salt is 140- 1410C. The isolation process described in the U. S. Patent No. 5,045,552 has numerous disadvantages such as large volume of solvents is required for azeotropic removal of water during which the product is exposed to high temperature and leads to certain impurities. Based on these drawbacks the isolation process finds to be unsuitable for preparation of amorphous rabeprazole sodium at commercial scale operations.
Japanese patent application JP 2001039975 indicates that the product obtained by example 33 of the U. S. Patent No. 5,045,552 with a melting point of
140-1410C corresponds to amorphous rabeprazole sodium. In this application, the X-ray powder diffraction pattern of the amorphous rabeprazole sodium is shown.
The PCT patent publication No. WO 03/101452 discloses a method for the preparation of rabeprazole sodium comprising dissolving rabeprazole base in aqueous sodium hydroxide and then subjecting to lyophilization. U.S. Patent No. 6,180,652 B1 (the ‘652 patent) describes acetone complex of rabeprazole sodium, process for its production and characterizes it by powder X-ray diffraction, infra-red spectroscopy and 1H-NMR spectroscopy. The ‘652 patent further reports a process for preparation of amorphous rabeprazole sodium by lyophilizing (freeze-drying) an aqueous solution of rabeprazole sodium acetone complex.
However, lyophilization is a technique, which is not suitable for production at industrial scale because this process presents serious limitations on cost, time, equipment capability and environmental protection.
According to PCT patent publication No. WO 2004/085424A1 , amorphous rabeprazole sodium is obtained by heating the rabeprazole sodium acetone complex at elevated temperature, preferably between 100 and 1100C. It is well known that exposing rabeprazole-type compounds to high temperatures increases the risk of decomposition to form impurities and as such, heat treatment of rabeprazole sodium acetone complex into amorphous rabeprazole sodium is not adequate for the production of a rabeprazole which is suitable for pharmaceutical use.
PCT patent publication No. WO 2007/023393 A2 reports a process for preparation of amorphous rabeprazole sodium, the said process comprises: i) contacting rabeprazole sodium acetone complex with a first solvent system which includes a hydrocarbon solvent or an ether solvent or an alcohol solvent or mixtures thereof; ii) filtering the solid from the solvent system used in step i) or distilling the solvent system used in step i) under reduced or atmospheric pressure, to thereby obtain a residue; iii) contacting the wet solid or the residue of step ii) with a second solvent system which includes a hydrocarbon solvent or an ether solvent; and iv) filtering to obtain a wet solid from the solvent system used in step iii) to obtain a wet solid.
The methods for preparation of amorphous rabeprazole sodium as described in the patents U.S. Patent No. 6,180,652 B1 , PCT patent publication No. WO 2004/085424A1 and PCT patent publication No. WO 2007/023393 A2 involves lengthy process i.e., proceeds via rabeprazole sodium acetone complex intermediate and also the yields obtained in these processes are very low.
U.S. Patent Application No. US2004/0180935A1 teaches a process for production of amorphous rabeprazole sodium by dissolving rabeprazole acid in a mixture of sodium hydroxide and methanol at 25-350C, removing the solvent by evaporation and precipitating the product by adding petroleum ether.
PCT patent publication No. WO 2006/120701 A1 teaches a process for manufacture of amorphous rabeprazole sodium with mean particle diameter between 10 to 55 μm, the said process comprises, addition of rabeprazole to aqueous sodium hydroxide; addition of ethyl alcohol to the solution; distillation of solvents from the solution thus obtained till thick mass is obtained; addition of an organic solvent selected from ethyl acetate, dichloromethane, chloroform, butyl acetate, ethanol, isopropyl alcohol, methanol, tetrahydrofuran, to the residue to obtain a clear solution; addition of this clear solution to an anti-solvent includes diisopropyl ether, diethyl ether, methyl tert-butyl ether, under agitation and isolation of the product.
Since a solvent may play an important role in increasing the yield rate or in determination of physical properties of drug substance such as crystal form, purity, solubility, etc., even if such a solvent is known to be toxic, there may be many cases that the use thereof in the preparation of drug substance cannot be avoided in terms of risk benefits. In such cases, this guideline (ICH guidelines Q3C(R3)) decrees that a concentration of a residual solvent in drug substance should be not more than a specified value, which is toxicologically acceptable. The methods for preparation of amorphous rabeprazole sodium as described in the patents, U.S. Patent Application No. US2004/0180935A1 and PCT patent publication No. WO 2006/120701 A1 suffers with residual solvent problem and thereby commercially not viable. These methods utilize the solvents like diisopropyl ether and petroleum ether as precipitating solvents. These solvents are difficult to remove completely by practical manufacturing techniques. According to the ICH guidelines Q3C(R3), there is no adequate toxicological data for the solvents like diisopropyl ether and petroleum ether on which to base a PDE was found. However, a need still remains for an improved and commercially viable process of preparing pure amorphous rabeprazole sodium that would solve the aforesaid problems associated with processes described in the prior art, which will be suitable for largr-scale preparation, in terms of simplicity, chemical yield and purity of the product, and which would carry out with comparatively smaller volume of solvent
]]>
Coenzyme Q10 (ubiquinone-10, CoQ10, CoQ, Q10 or simply Q) is aubiquinone containing 10 isoprenoid units. First discovered in 1957 by Crane et al. [1], its chemical structure was determined by Karl Folkers [2], who later won the Priestley medal from the American Chemical Society. This oil-soluble, vitamin-like micronutrient forms part of the electron transport chain which, in the process of aerobic respiration, generates 95% of the human body’s energy asATP [3].
CoQ, or Q10 is a 1,4-benzoquinone, where Q refers to the quinone chemical group, and 10 refers to the number of isoprenylchemical subunits in its tail.
This oil-soluble, vitamin-like substance is present in most eukaryotic cells, primarily in themitochondria. It is a component of the electron transport chain and participates in aerobic cellular respiration, generating energy in the form of ATP. Ninety-five percent of the human body’s energy is generated this way. Therefore, those organs with the highest energy requirements—such as the heart, liver and kidney—have the highest CoQ10concentrations. There are three redox states of CoQ10: fully oxidized (ubiquinone), semiquinone (ubisemiquinone), and fully reduced (ubiquinol). The capacity of this molecule to exist in a completely oxidized form and a completely reduced form enables it to perform its functions in the electron transport chain and as an antioxidant respectively.
Coenzyme Q10 is synthesized de novo by every cell in the body, but levels decrease with age, in several clinical disorders, and in patients administered certain drugs such as hydroxymethylglutaryl-CoA reductase inhibitors (commonly known as statins). With cardiovascular disease being a leading cause of death in the West, evidence that oral supplements of coenzyme Q10 can benefit patients suffering from heart disease is of increasing appeal. Evidence is also accumulating for its effective treatment of other ailments including mitochondrial disorders and neurodegenerative diseases such as amyotrophic lateral sclerosis (ALS), Huntington’s disease and Parkinson’s disease.
Coenzyme Q10 is one of the best-selling dietary supplements worldwide, available over the counter from health food shops and pharmacies. Its popularity may be due to the wide-ranging claims made for its effectiveness in a myriad of human health issues: it is marketed as an energy booster; a periodontal health promoter; an agent for maintaining normal blood-cholesterol levels; an enhancer of cognitive function; a remedy for hypertension, migraine headaches, radiation injury and cancer; and a superdrug capable of delaying or even reversing the effects of aging. However, perusal of the scientific literature reveals that, while data supporting some claims are forthcoming (such as in the case of heart disease and mitochondrial function), coenzyme Q10 is neither panacea nor elixir [4,5].
References
- Crane, F.L., Hatefi, Y., Lester, R.L. and Widmer, C. (1957) Isolation of a quinone from beef heart mitochondria. Biochim. Biophys. Acta 25, 220–221.
- Wolf, D.E., Hoffman, C.H., Trenner, N.R., Arison, B.H., Shunk, C.H., Linn, B.O., McPherson, J.F. and Folkers, K. (1958) Coenzyme Q. I. Structure studies on the coenzyme Q group. J.Am. Chem. Soc. 80, 4752.
- Ernster, L. and Dallner, G. (1995) Biochemical, physiological and medical aspects of ubiquinone function. Biochim. Biophys.Acta 1271, 195–204.
- Watts, T.L. (1995), Coenzyme Q10 and periodontal treatment: is there any beneficial effect? Br. Dent. J. 178, 209–213.
- European Food Safety Authority Panel on Dietetic Products, Nutrition and Allergies (2010), Scientific Opinion on the substantiation of health claims related to coenzyme Q10 and contribution to normal energy-yielding metabolism (ID 1508, 1512, 1720, 1912, 4668), maintenance of normal blood pressure (ID 1509, 1721, 1911), protection of DNA, proteins and lipids from oxidative damage (ID 1510), contribution to normal cognitive function (ID 1511), maintenance of normal blood cholesterol concentrations (ID 1721) and increase in endurance capacity and/or endurance performance (ID 1913) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J. 8, 1793–1819.

ENJOY BLOG
BY
DR ANTHONY MELVIN CRASTO Ph.D , Born in Mumbai in 1964 and graduated from Mumbai University, Completed his PhD from ICT ,1991, Mumbai, India in Organic chemistry, The thesis topic was Synthesis of Novel Pyrethroid Analogues,
Currently he is working with GLENMARK- GENERICS LTD, Research centre as Principal Scientist, Process Research (bulk actives) at Mahape, Navi Mumbai, India.
Prior to joining Glenmark, he worked with major multinationals like Hoechst Marion Roussel, now Sanofi Aventis, & Searle India ltd, now Rpg lifesciences, etc. He has worked in Basic research, Neutraceuticals, Natural products, Flavors, Fragrances, Pheromones, Vet Drugs, Drugs, formulation, GMP etc. He has total 25 yrs exp in this field, he is now helping millions, has million hits on google on all organic chemistry websites.
His New Drug Approvals , Green Chemistry International, Eurekamoments in Organic Chemistry , Organic Chemistry by Dr Anthony, WIX BLOG , are some most read chemistry blogs
He has hands on experience in initiation and developing novel routes for drug molecules and implementation them on commercial scale over a 25 year tenure, good knowledge of IPM, GMP, Regulatory aspects, he has several international drug patents published worldwide .
He has good proficiency in Technology Transfer, Spectroscopy , Stereochemistry , Synthesis, Reactions in Org Chem , Polymorphism, Pharmaceuticals , Medicinal chemistry , Organic chemistry literature , Patent related site , Green chemistry , Reagents , R & D , Molecules , Heterocyclic chem , Sourcing etc
He suffered a paralytic stroke in dec 2006 and is bound to a wheelchair, this seems to have injected feul in him to help chemists around the world, he is more active than before and is pushing boundaries, he has one lakh connections on all networking sites, He makes himself available to all, contact him on +91 9323115463, amcrasto@gmail.com
]]>CHECK OUT MY BLOG NEW DRUG APPROVALS AT
https://newdrugapprovals.wordpress.com/
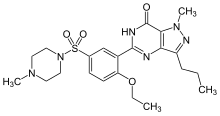
sildanafil
Erection of the penis in males is often a result of a state of sexual arousal. Erectile dysfunction occurs when it becomes difficult to produce erection even in a state of adequate arousal. Erectile dysfunction can occur at any age to any one and at any point of time. It can be due to a vast array of reasons, ranging from fatigue to serious diabetic or heart conditions. While causes like fatigue can be taken care of by simple rest and a good night’s sleep, serious causes like diabetes and cardiovascular diseases can be a little difficult to deal with. Erectile dysfunction does not necessarily mean that there is something physically wrong within the body, as it can also be a result of a vast number of psychological reasons. The loss of erection in itself can give rise to a vast number of psychological problems like loss of self respect and confidence and, hence, requires immediate medical assistance.

vardenafil
You can characterize erectile dysfunction (also known as the problem of male impotency) into two broad categories: firstly, when sometimes full erections are obtained, like when the person under consideration is in deep sleep. This condition is due to the failure of getting an erection due to a psychological reason and can be solved with professional psychological assistance. Secondly, when no erection is obtained. This is generally when the physical structure is not working properly.

Erectile dysfunction takes place when a man fails to get a proper erection or is not able to sustain it to indulge in sexual intercourse. There is no formal means of detecting and diagnosing an erectile dysfunction. However, blood tests are conducted in such cases as they generally give a fair idea of the underlying diseases such as prolactinoma, diabetes and hypogonadism. Impotency is generally a result of poor health conditions and can be a result of obesity or malnutrition. There are a number of tests along with the blood tests that are undertaken to determine the nature and extent of an erectile dysfunction problem. These are duplex ultrasound to evaluate the blood flow, penile nervous function test such as bulbocavernosus reflex, nocturnal penile tumescence, penile biothesiometry, Magnetic Resonance Angiography (MRA), etc.
Some patients have trouble discussing problems relating to erectile dysfunction with their doctors, but it is important to step forward as erectile dysfunction can also be a symptom of other health problems such as clogged arteries or nerve damage. A doctor can offer a number of treatments for erectile dysfunction depending on the reason and underlying conditions.
While some treatments may involve a steady intake of medicines over a period of time, others can be as simple as taking a few pills for some days and getting more exercise and physical activity. The treatment generally lasts for about a month, but can also be of shorter or longer duration, depending on the severity of the disorder. If the erectile dysfunction is due to some other major ailment, then the problem generally subsides after complete recovery.
When a patient is suffering from erectile dysfunction, he generally has a very low self esteem and, hence, it becomes important that he get professional help and doesn’t try to deal with the situation all by himself. Becoming a part of a support group and taking psychological help from a psychiatrist often helps.
The most common medicines prescribed for erectile dysfunctions are sildanafil or viagra, vardenafil or levitra, and tadalafil or cialis. These medicines can cause side effects such as dizziness and headaches, and should be only taken under expert medical supervision. Some of the other side effects of these medicines may include an increased blood pressure and, thus, are not recommended for heart patients.
Remedies for Erectile Dysfunction
Here are several natural remedies that are used for erectile dysfunction.
L-Arginine
L-arginine is an amino acid that the body uses to make nitric oxide, a substance signals smooth muscle surrounding blood vessels to relax, which dilates the blood vessels and increases blood flow. Relaxation of smooth muscle in the penis allows for enhanced blood flow, leading to an erection.
L-arginine is found naturally in foods such as meat, dairy, poultry and fish. It is also available as oral L-arginine supplements, which some product manufacturers market as a “natural Viagra”).
There have only been two studies to date, however, evaluating the effectiveness of L-arginine for erectile dysfunction.
One study involved 50 men who took L-arginine (5 grams a day) or a placebo. After six weeks, significantly more men taking L-arginine experienced an improvement in sexual function compared with men taking the placebo. Interestingly, it only benefited men who had initially low levels of nitric oxide.
Another study using a smaller dose of L-arginine and a shorter treatment duration found no benefit with L-arginine use. The study involved 32 men with erectile dysfunction who took oral L-arginine supplements (500 milligrams three times per day) or a placebo for 17 days. Oral L-arginine was no better than the placebo.
Side effects may include digestive complaints. High dosees of L-arginine may stimulate the body’s production of gastrin, a hormone that increases stomach acid. For this reason, L-arginine may be harmful for individuals with ulcers and people taking drugs that are hard on the stomach.
L-arginine may also alter potassium levels in the body, especially in people with liver disease. It should not be taken by people who are on medications that alter potassium levels, such as potassium sparing diuretics and ACE inhibitors
Propionyl-L-Carnitine
One study examined the use of two forms of carnitine, propionyl-L-carnitine and acetyl-L-carnitine in 96 men who with erectile dysfunction after prostate surgery. One group were given a placebo, another group took propionyl-L-carnitine (2 grams per day) plus acetyl-L-carnitine (2 grams per day) and sildenafil (Viagra) when needed, and the third group used Viagra alone.
Propionyl-L-carnitine and acetyl-L-carnitine were found to enhance the effectiveness of sildenafil, and result in improved erectile function, sexual intercourse satisfaction, orgasm, and general sexual well-being compared to Viagra alone.
Another study examined the effectiveness of propionyl-L-carnitine supplements plus sildenafil in men with erectile dysfunction and diabetes who were previously unresponsive to Viagra alone. Participants in the study received either propionyl-L-carnitine (two grams per day) plus Viagra (50 milligrams twice a week) or Viagra alone. After 24 weeks, propionyl-L-carnitine plus Viagra was significantly more effective than Viagra alone.
Gingko
The herb ginkgo is used for erectile dysfunction, particularly in people who experience sexual dysfunction as a side effect of antidepressant drugs. It appears to relax smooth muscle and enhance blood flow in the penis.

In one study of 60 men with erectile dysfunction, there was a 50 percent success rate after six months of ginkgo treatment. Two additional studies, however, found that ginkgo was no better than a placebo.
Zinc
Siginificant depletion of the mineral zinc, associated with long-term use of diuretics, diabetes, digestive disorders, and certain kidney and liver diseases, has been shown to lead to erectile dysfunction.
Ashwagandha

The herb ashwagandha (Withania somnifera) is sometimes called Indian Ginseng because it is thought to have similar effects on the body. It is thought to increase energy, stamina, and sexual function. No studies, however, have examined whether it is effective for erectile dysfunction in humans.
Side effects of ashwagandha may include drowsiness. It should not be combined with sedative drugs.
Yohimbe
The bark of the west African yohimbe tree is a source of yohimbine, a compound that has been found to stimulate blood flow to the penis, increase libido, and decrease the period between ejaculations.

Yohimbe is not recommended, however, because it is potentially dangerous, even in small doses. Side effects may include dizziness, anxiety, nausea, a severe drop in blood pressure, abdominal pain, fatigue, hallucinations, and paralysis.
Tongkat Ali
Tongkat Ali was dubbed the “Asian Viagra” in a May 1999 report in the New Sunday Times.
It has been used in Malaysia for many years by men to increase sexual desire, libido, sexual performance and to treat erectile dysfunction.
Tongkat ali appears to work by increasing levels of the hormone testosterone. Testosterone is primarily responsible for the growth and development of male reproductive organs, including the penis, testicles, scrotum, prostate, and seminal vesicles. Normal testosterone levels maintain energy level, mood, fertility, and sexual desire.
Because of its testosterone-enhancing properties, tongkat ali is also used by bodybuilders to increase muscle mass and strength
…………………………………………………………………………………………………..
Tribulus terrestris

Tribulus terrestris, also known as puncture vine, is a herb that has been used in the traditional medicine of China and India for centuries.
In the mid-1990s, tribulus terrestris became known in North America after Eastern European Olympic athletes said that taking tribulus helped their performance.
The active compounds in tribulus are called steroidal saponins. Two types, called furostanol glycosides and spirostanol glycosides, appear to be involved with the effects of tribulus. These saponins are found primarily in the leaf.
Tribulus is most often used for infertility, erectile dysfunction, and low libido. In the last decade, it has become popular to improve sports performance.
Tribulus has been marketed these conditions because research performed in Bulgaria and Russia indicates that tribulus increases levels of the hormones testosterone (by increasing luteinizing hormone), DHEA, and estrogen. The design of these research studies, however, has been questioned.
A more recent study found that four weeks of tribulus supplements (at 10 to 20 milligrams per kg of body weight daily) had no effect on male sex hormones testosterone, androstenedione, or luteinizing hormone compared to people who did not take tribulus.
Preliminary animal studies found that tribulus heightened sexual behavior and increased intracavernous pressure. This was attributed to increases in testosterone. There haven’t been any well-designed human studies to confirm these early findings.
…………………………………………………………………………………………………………………….
Maca
Maca (Lepidium meyenii) is a root plant consumed as a food and for medicinal purposes. Maca is also known as “Peruvian ginseng” (despite the fact that it is not a member of the ginseng family), because it is used as a folk remedy to increase stamina, energy, and sexual function. It is typically taken as a pill or liquid extract or as powdered maca root.
Long used to enhance energy and boost stamina, maca is often touted as an aphrodisiac and a natural means of improving sexual performance and fertility. Although few scientific studies have tested maca’s medicinal effects, some research suggests that maca may offer certain health benefits.
Proponents claim that maca may help with these health concerns:
- fatigue
- infertility
- symptoms of menopause
- sexual dysfunction in women
- sexual dysfunction in men (including erectile dysfunction)
Maca is also said to aid in the treatment of cancer.
Here’s a look at the available research on maca and its potential health benefits:
There is “limited evidence” for maca’s effectiveness in improving sexual function in men and women, according to a 2010 report published in BMC Complementary and Alternative Medicine. The report’s authors analyzed four clinical trials, two of which found that maca may have positive effects on sexual dysfunction or sexual desire in healthy menopausal women or healthy adult men. However, the other two trials found that maca failed to produce any positive effects on sexual function.
In a 2008 study from CNS Neuroscience & Therapeutics, researchers found that maca may help alleviate sexual dysfunction caused by use of selective-serotonin reuptake inhibitors (or SSRIs, a class of medications used in treatment of depression). The study involved 20 people with depression, all of whom were experiencing SSRI-induced sexual dysfunction. Results revealed that maca may also help improve libido.
………………………………………………………………………………………………………………
Muira Palma
Muira puama is a small Brazilian tree that grows across the Amazon river basin. It has a long history of use in Brazilian folk medicine as an aphrodisiac.
The root and stem of the tree are used medicinally.
Muira puama is used mainly as a herbal remedy for erectile dysfunction and sexual dysfunction in women.
……………………………………………………………………………………………………………..
Damiana
Although damiana contains about 1/10 of the arbutin as the herb uva ursi, a maximum safe dose of damiana has not been established.
The safety of damiana in children, pregnant or nursing women, or people with liver or kidney disease has not been established.
…………………………………………………………………………………………………………..
Fo-Ti

Other Names: Polygonum multiflorum, He shou wu
Fo-ti is a plant native to China that is also found in Japan and Taiwan. The medicinal part of the plant is the root. In traditional Chinese medicine, it is often boiled in a liquid made with black beans — this is known as red fo-ti. White fo-ti is the unprocessed root.
Fo-ti is called He shou wu, which means “black-haired Mr. He” in Chinese. This name refers to a legend of an older villager named Mr. He who took fo-ti and restored his black hair, youthful appearance and vitality.
……………………………………………………………………………………………………………….
Horny Goat Weed
Horny goat weed is a leafy plant that is native to Asia and the Mediterranean region. It is also known as Epimedium and Yin Yan Huo.
Horny goat weed has a long history of use in traditional Chinese medicine.
According to folklore, horny goat weed’s reputed aphrodisiac qualities were discovered when a Chinese goat herder noticed increased sexual activity in his flock after they ingested the weed.
Animal studies indicate that horny goat weed may work by increasing nitric oxide levels, which relaxes smooth muscle and lets more blood flow to the penis or clitoris.
Horny goat weed also appears to act by inhibiting the PDE-5 enzyme, which is the same way that the popular drug Viagra works.
Some evidence suggests horny goat weed may modulate levels of the hormones cortisol, testosterone, and thyroid hormone, bringing low levels back to normal.
]]>