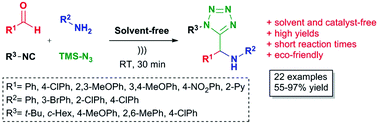
Originally posted on ORGANIC CHEMISTRY SELECT: ? Endogenous water-triggered and ultrasound accelerated synthesis of 1,5-disubstituted tetrazoles via a solvent and catalyst-free Ugi-azide reaction Green Chem., 2017, Advance Article DOI: 10.1039/C6GC03324E, Communication Shrikant G. Pharande, Alma Rosa Corrales Escobosa, Rocio Gamez-Montano An ultrasound accelerated, environmentally benign Ugi-azide based method was developed for the synthesis of 1,5-disubstituted…
Endogenous water-triggered and ultrasound accelerated synthesis of 1,5-disubstituted tetrazoles via a solvent and catalyst-free Ugi-azide reaction
DOI: 10.1039/C6GC03324E, Communication
An ultrasound accelerated, environmentally benign Ugi-azide based method was developed for the synthesis of 1,5-disubstituted tetrazoles under solvent and catalyst-free conditions.
Endogenous water-triggered and ultrasound accelerated synthesis of 1,5-disubstituted tetrazoles via a solvent and catalyst-free Ugi-azide reaction
E-mail: rociogm@ugto.mx
DOI: 10.1039/C6GC03324E, http://pubs.rsc.org/en/Content/ArticleLanding/2017/GC/C6GC03324E?utm_source=feedburner&utm_medium=feed&utm_campaign=Feed%3A+rss%2FGC+%28RSC+-+Green+Chem.+latest+articles%29#!divAbstract


//////////////