The Pinnick oxidation is also known as Lindgren oxidation. It is an organic reaction by which aldehydes can be oxidized into its corresponding carboxylic acid, originally developed by Lindgren and Nilsson.ref1 The typical reaction condition used today was modified by G. A. Kraus even before Pinnick.ref2,3 Pinnick proved this condition as general. There are number of ways to oxidize the aldehydes however, only few reactions are endurable to the broad range of functional groups. One of such reaction is Pinnick Oxidation which can provide preferred transformation even to systems that contain sensitive functionalities or sterically hindered groups. This reaction is especially useful for oxidizing α-methylene aldehyde units.ref4 It is an inexpensive method for effectively oxidizing aldehydes instereospecific manner. The reaction is named after chemist H.W. Pinnick.
|
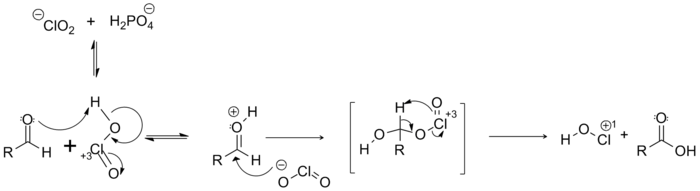
Mechanism
|
Following reaction mechanism was proposed for Pinnick Oxidation. However, the mechanism follows formation of a by-product that can cause a side reaction which can inevitably consume NaClO2. Therefore, the reaction requires the use of scavengers to be able to remove the by-products.6

Synthetic Uses
Zaragozic acid is a potent squalene synthase inhibitor that can reduce the side effects caused by cholesterol inhibiting drugs which can also interfere with the production of important steroids. The total synthesis of Zaragozic acid applied by A. Armstrong was possible through Pinnick Oxidation. Different oxidation methods such as Jones oxidation, modified Ley Oxidation were also applied. However, the efforts resulted in a mixture of products. A good yield with good purity could be obtained via Pinnick Oxidation.
References
- Lindgren, Bengt O.; Nilsson, Torsten; Husebye, Steinar; Mikalsen, ØYvind; Leander, Kurt; Swahn, Carl-Gunnar (1973). “Preparation of Carboxylic Acids from Aldehydes (Including Hydroxylated Benzaldehydes) by Oxidation with Chlorite”. Acta Chemica Scandinavica 27: 888. doi:10.3891/acta.chem.scand.27-0888.
- George A. Kraus; Bruce Roth (1980). “Synthetic studies toward verrucarol. 2. Synthesis of the AB ring system”. The Journal of Organic Chemistry 45: 4825. doi:10.1021/jo01312a004.
- George A. Kraus; Michael J. Taschner (1980). “Model studies for the synthesis of quassinoids. 1. Construction of the BCE ring system”.The Journal of Organic Chemistry 45: 1175. doi:10.1021/jo01294a058.
- Bal, B. S.; Childers, W.E.; Pinnick, H.W. (1981). “Oxidation of α,β-Unsaturated Aldehydes”. Tetrahedron 37: 2091–2096.doi:10.1016/S0040-4020(01)97963-3.
- Mundy, B.J; Michael G. Ellerd, Frank G. Favaloro (2005). Name reactions and reagents in organic synthesis. John Wiley & Sons. ISBN 0-471-22854.
6 Corey, E.J; K.C. Nicolaou (2005). Strategic Applications of Named Reactions. Elsevier, Inc. ISBN 978-7-03-019190-8.