Avanafil is a PDE5 inhibitor approved for erectile dysfunction on April 27, 2012.[1] Avanafil is known by the trademark name Stendra and was developed by Vivus Inc. It acts by inhibiting a specific phosphodiesterase type 5 enzyme which is found in various body tissues, but primarily in the corpus cavernosum penis, as well as the retina. Other similar drugs are sildenafil, tadalafil and vardenafil. The advantage of avanafil is that it has very fast onset of action compared with other PDE5 inhibitors.
 |
|||
|---|---|---|---|
|
|||
SynthesisAvanafil can be synthesized from a benzylamine derivative and a pyrimidine derivative:[2]
|
|||
 links
links
- Faster-Working Erectile Dysfunction Drug?. CBS News. November 24, 2009.
- Vivus says men taking avanafil were more likely to be ready for sex within 15 minutes. The Gaea Times. January 11, 2010.
- “Avanafil is the New Player in The Erectile Dysfunction Field”. June 28, 2011
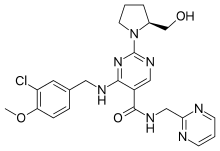
- NAME—4-[(3-Chloro-4-methoxybenzyl)amino]-2-[(2S)-2-(hydroxymethyl)-1-p
yrrolidinyl]-N-(2-pyrimidinylmethyl)-5-pyrimidinecarboxamide

