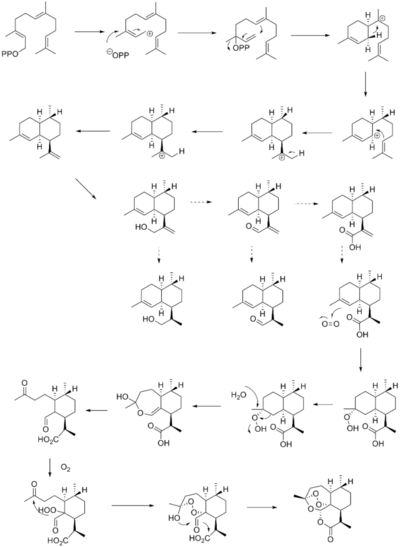
Total synthesis of artemisinin, WO2012154906 (A1) 15th nov 2012
link at espacenet http://worldwide.espacenet.com/publicationDetails/biblio?DB=EPODOC&II=0&ND=3&adjacent=true&locale=en_EP&FT=D&date=20121115&CC=WO&NR=2012154906A1&KC=A1
Indiana University Research And Technology Corporation
| Inventors | Cook, Silas; Zhu, Chun-Yin |
|---|
| Application number: | WO2012US37214 2012 05 10 |
|---|---|
| Priority number(s): | US201161484317P 2011 05 10 |
Oxidative synthesis; Rearrangement synthesis, The present invention provides a method for manufacturing artemisinin and its congeners from cyclohexenone as a starting material.
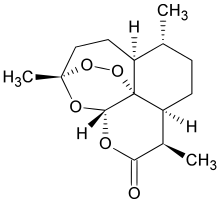 |
|
|---|---|
| Systematic (IUPAC) name | |
| (3R,5aS,6R,8aS,9R,12S,12aR)- octahydro-3,6,9-trimethyl-3,12- epoxy-12H-pyrano[4,3-j]- 1,2-benzodioxepin-10(3H)-oneArtemisinin also known as Qinghaosu (Chinese: 青蒿素), and its derivatives are a group of drugs that possess the most rapid action of all current drugs against Plasmodium falciparum malaria.[1] Treatments containing an artemisinin derivative (artemisinin-combination therapies, ACTs) are now standard treatment worldwide for P. falciparum malaria. The starting compound artemisinin is isolated from the plant Artemisia annua, sweet wormwood, a herb employed in Chinese traditional medicine. Chemically, artemisinin is a sesquiterpene lactone containing an unusual peroxide bridge. This peroxide is believed to be responsible for the drug’s mechanism of action. Few other natural compounds with such a peroxide bridge are known.[2] (Ascaridole is another.) Use of the drug by itself as a monotherapy is explicitly discouraged by the World Health Organization, as there have been signs that malarial parasites are developing resistance to the drug. Therapies that combine artemisinin with some other antimalarial drug are the preferred treatment for malaria and are both effective and well tolerated in patients. The drug is also increasingly being used in Plasmodium vivax malaria,[3] as well as being a topic of research in cancer treatment. References
biosyn
|