 Epoxidation
Epoxidation
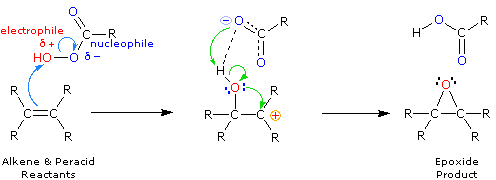 |
 OZONOLYSIS
OZONOLYSIS
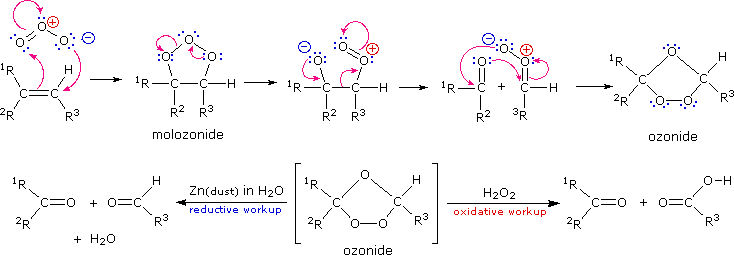
 oxidations
oxidations
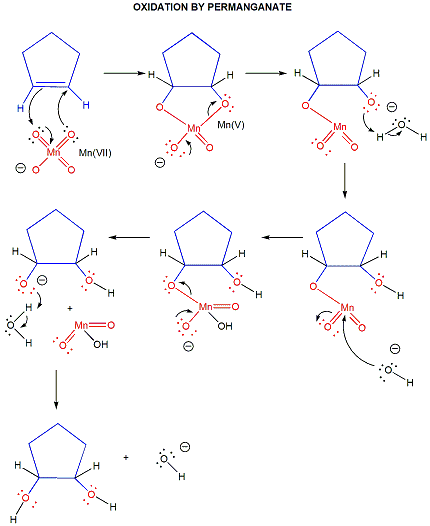
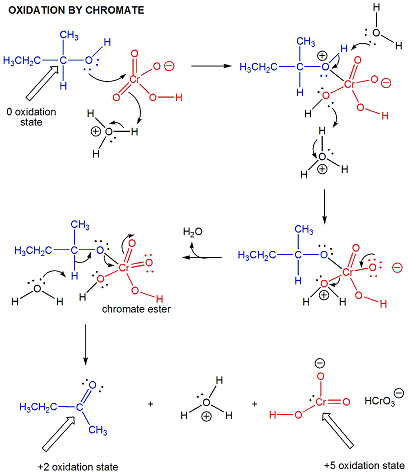
 DIELS ALDER RXN
DIELS ALDER RXN
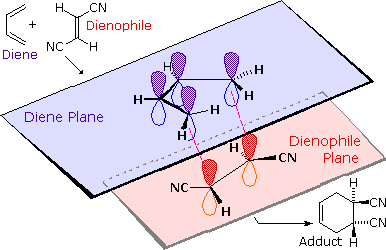
We noted earlier that addition reactions of alkenes often exhibited STEREOSPEFICITY, in that the reagent elements in some cases added syn and in other cases anti to the the plane of the double bond. Both reactants in the Diels-Alder reaction may demonstrate stereoisomerism, and when they do it is found that the relative configurations of the reactants are preserved in the product (the adduct). The following drawing illustrates this fact for the reaction of 1,3-butadiene with (E)-dicyanoethene. The trans relationship of the cyano groups in the dienophile is preserved in the six-membered ring of the adduct. Likewise, if the terminal carbons of the diene bear substituents, their relative configuration will be retained in the adduct. Using the earlier terminology, we could say that bonding to both the diene and the dienophile is syn. An alternative description, however, refers to the planar nature of both reactants and terms the bonding in each case to be suprafacial (i.e. to or from the same face of each plane). This STEREOSPECITY also confirms the synchronous nature of the 1,4-bonding that takes place
 NITRILE HYDROLYSIS UNDER ACIDIC CONDITIONS
NITRILE HYDROLYSIS UNDER ACIDIC CONDITIONS

 NITRILE HYDOLYSIS, BASIC CONDITIONS
NITRILE HYDOLYSIS, BASIC CONDITIONS
