Levoglucosenone
Levoglucosenone is a highly dehydrated sugar that has been used in the preparation of chiral synthons such as (–)-y-multistriatin and (+)-Prelog—Djerassi lactonic acid.
Negative-form is a pyrolysis product of cellulose and cellulose-containing materials including pulp and paper waste products. Known as a pyrolytic product of cellulose, which is very useful as a chiral source for synthesizing natural products.

Levoglucosenone
| Density | 1.29 g/mL |
|---|---|
| Boiling Point | 57-57.5 °C / 0.5 mm |
|
|||||||||||||
|
|||||||||||||
also Taniguchi, T., et al.: Synlett, 971 (1996),
Efficient levoglucosenone production
DOI: 10.1039/C1GC15975E
http://pubs.rsc.org/en/Content/ArticleLanding/2011/GC/c1gc15975e COPY PASTE LINK
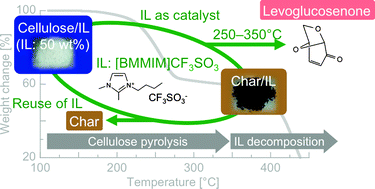


See papers on levoglucosenone:
(1S,5R)-6,8-Dioxabicyclo[3.2.1]oct-2-en-4-one
M.S.Miftakhov, et al.
Levoglucosenone: the properties, reactions, and use in fine organic synthesis
Russ.Chem.Rev., 1994, 63 (10), 869-882
Herdewijn P, et al.
3′-substituted 2′,3′-dideoxynucleoside analogues as potential anti-HIV (HTLV-III/LAV) agents
J.Med.Chem., 1987, 30(8), 1270-1278
M.Jung and M.Kiankarimi
Synthesis of Methylene-Expanded 2′,3′-Dideoxyribonucleosides
J.Org.Chem., 1998, 63 (23), 8133-8144
http://levoglucosenone.com/derivatives/ COPY PASTE LINK